Injection of IK1 through dynamic clamp can make all the difference in patch-clamp studies on hiPSC-derived cardiomyocytes
- PMID: 38152247
- PMCID: PMC10751953
- DOI: 10.3389/fphys.2023.1326160
Injection of IK1 through dynamic clamp can make all the difference in patch-clamp studies on hiPSC-derived cardiomyocytes
Abstract
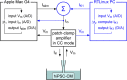
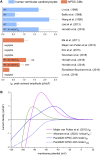
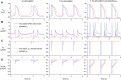
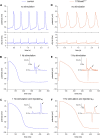
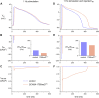
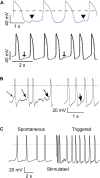
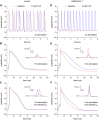
Human-induced stem cell-derived cardiomyocytes (hiPSC-CMs) are a valuable tool for studying development, pharmacology, and (inherited) arrhythmias. Unfortunately, hiPSC-CMs are depolarized and spontaneously active, even the working cardiomyocyte subtypes such as atrial- and ventricular-like hiPSC-CMs, in contrast to the situation in the atria and ventricles of adult human hearts. Great efforts have been made, using many different strategies, to generate more mature, quiescent hiPSC-CMs with more close-to-physiological resting membrane potentials, but despite promising results, it is still difficult to obtain hiPSC-CMs with such properties. The dynamic clamp technique allows to inject a current with characteristics of the inward rectifier potassium current (IK1), computed in real time according to the actual membrane potential, into patch-clamped hiPSC-CMs during action potential measurements. This results in quiescent hiPSC-CMs with a close-to-physiological resting membrane potential. As a result, action potential measurements can be performed with normal ion channel availability, which is particularly important for the physiological functioning of the cardiac SCN5A-encoded fast sodium current (INa). We performed in vitro and in silico experiments to assess the beneficial effects of the dynamic clamp technique in dissecting the functional consequences of the SCN5A-1795insD+/- mutation. In two separate sets of patch-clamp experiments on control hiPSC-CMs and on hiPSC-CMs with mutations in ACADVL and GNB5, we assessed the value of dynamic clamp in detecting delayed afterdepolarizations and in investigating factors that modulate the resting membrane potential. We conclude that the dynamic clamp technique has highly beneficial effects in all of the aforementioned settings and should be widely used in patch-clamp studies on hiPSC-CMs while waiting for the ultimate fully mature hiPSC-CMs.
Keywords: GNB5; SCN5A; acetylcholine-activated potassium current; delayed afterdepolarizations; fast sodium current; inward rectifier potassium current; triggered action potentials; ventricular action potential.
Copyright © 2023 Verkerk and Wilders.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: a dynamic clamp study with virtual IK1.Front Physiol. 2015 Feb 3;6:7. doi: 10.3389/fphys.2015.00007. eCollection 2015. Front Physiol. 2015. PMID: 25691870 Free PMC article.
-
Patch-Clamp Recording from Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Improving Action Potential Characteristics through Dynamic Clamp.Int J Mol Sci. 2017 Aug 30;18(9):1873. doi: 10.3390/ijms18091873. Int J Mol Sci. 2017. PMID: 28867785 Free PMC article.
-
Automated Dynamic Clamp for Simulation of IK1 in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Real Time Using Patchliner Dynamite8.Curr Protoc Pharmacol. 2020 Mar;88(1):e70. doi: 10.1002/cpph.70. Curr Protoc Pharmacol. 2020. PMID: 31868992
-
Biophysical comparison of sodium currents in native cardiac myocytes and human induced pluripotent stem cell-derived cardiomyocytes.J Pharmacol Toxicol Methods. 2018 Mar-Apr;90:19-30. doi: 10.1016/j.vascn.2017.11.001. Epub 2017 Nov 8. J Pharmacol Toxicol Methods. 2018. PMID: 29128504
-
Dynamic Clamp in Electrophysiological Studies on Stem Cell-Derived Cardiomyocytes-Why and How?J Cardiovasc Pharmacol. 2021 Mar 1;77(3):267-279. doi: 10.1097/FJC.0000000000000955. J Cardiovasc Pharmacol. 2021. PMID: 33229908 Review.
Cited by
-
Modeling the atrioventricular conduction axis using human pluripotent stem cell-derived cardiac assembloids.Cell Stem Cell. 2024 Nov 7;31(11):1667-1684.e6. doi: 10.1016/j.stem.2024.08.008. Epub 2024 Sep 10. Cell Stem Cell. 2024. PMID: 39260368 Free PMC article.
-
SCN10A-short gene therapy to restore conduction and protect against malignant cardiac arrhythmias.Eur Heart J. 2025 May 7;46(18):1747-1762. doi: 10.1093/eurheartj/ehaf053. Eur Heart J. 2025. PMID: 39973098 Free PMC article.
-
Alleviating the Effects of Short QT Syndrome Type 3 by Allele-Specific Suppression of the KCNJ2 Mutant Allele.Int J Mol Sci. 2024 Dec 12;25(24):13351. doi: 10.3390/ijms252413351. Int J Mol Sci. 2024. PMID: 39769116 Free PMC article.
-
Chronic Mexiletine Administration Increases Sodium Current in Non-Diseased Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes.Biomedicines. 2024 May 29;12(6):1212. doi: 10.3390/biomedicines12061212. Biomedicines. 2024. PMID: 38927420 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

