Characterizing atherosclerotic tissues: in silico analysis of mechanical properties using intravascular ultrasound and inverse finite element methods
- PMID: 38152285
- PMCID: PMC10751321
- DOI: 10.3389/fbioe.2023.1304278
Characterizing atherosclerotic tissues: in silico analysis of mechanical properties using intravascular ultrasound and inverse finite element methods
Abstract
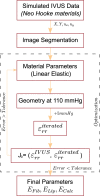
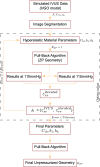
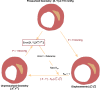
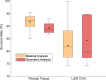
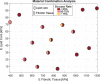
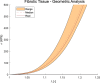
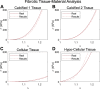
Atherosclerosis is a prevalent cause of acute coronary syndromes that consists of lipid deposition inside the artery wall, creating an atherosclerotic plaque. Early detection may prevent the risk of plaque rupture. Nowadays, intravascular ultrasound (IVUS) is the most common medical imaging technology for atherosclerotic plaque detection. It provides an image of the section of the coronary wall and, in combination with new techniques, can estimate the displacement or strain fields. From these magnitudes and by inverse analysis, it is possible to estimate the mechanical properties of the plaque tissues and their stress distribution. In this paper, we presented a methodology based on two approaches to characterize the mechanical properties of atherosclerotic tissues. The first approach estimated the linear behavior under particular pressure. In contrast, the second technique yielded the non-linear hyperelastic material curves for the fibrotic tissues across the complete physiological pressure range. To establish and validate this method, the theoretical framework employed in silico models to simulate atherosclerotic plaques and their IVUS data. We analyzed different materials and real geometries with finite element (FE) models. After the segmentation of the fibrotic, calcification, and lipid tissues, an inverse FE analysis was performed to estimate the mechanical response of the tissues. Both approaches employed an optimization process to obtain the mechanical properties by minimizing the error between the radial strains obtained from the simulated IVUS and those achieved in each iteration. The second methodology was successfully applied to five distinct real geometries and four different fibrotic tissues, getting median R 2 of 0.97 and 0.92, respectively, when comparing the real and estimated behavior curves. In addition, the last technique reduced errors in the estimated plaque strain field by more than 20% during the optimization process, compared to the former approach. The findings enabled the estimation of the stress field over the hyperelastic plaque tissues, providing valuable insights into its risk of rupture.
Keywords: atherosclerosis; coronary; inverse finite element analysis; material characterization; optimization; vulnerability.
Copyright © 2023 Latorre, Martínez and Peña.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
A Framework for Local Mechanical Characterization of Atherosclerotic Plaques: Combination of Ultrasound Displacement Imaging and Inverse Finite Element Analysis.Ann Biomed Eng. 2016 Apr;44(4):968-79. doi: 10.1007/s10439-015-1410-8. Epub 2015 Sep 23. Ann Biomed Eng. 2016. PMID: 26399991 Free PMC article.
-
Patient specific characterization of artery and plaque material properties in peripheral artery disease.J Mech Behav Biomed Mater. 2020 Jan;101:103453. doi: 10.1016/j.jmbbm.2019.103453. Epub 2019 Sep 27. J Mech Behav Biomed Mater. 2020. PMID: 31585351 Free PMC article.
-
Mechanical Characterization of the Vessel Wall by Data Assimilation of Intravascular Ultrasound Studies.Front Physiol. 2018 Mar 28;9:292. doi: 10.3389/fphys.2018.00292. eCollection 2018. Front Physiol. 2018. PMID: 29643815 Free PMC article.
-
Review: Mechanical Characterization of Carotid Arteries and Atherosclerotic Plaques.IEEE Trans Ultrason Ferroelectr Freq Control. 2016 Oct;63(10):1613-1623. doi: 10.1109/TUFFC.2016.2572260. Epub 2016 May 26. IEEE Trans Ultrason Ferroelectr Freq Control. 2016. PMID: 27249826 Review.
-
Intravascular ultrasound elastography: an overview.Ultrasonics. 2002 May;40(1-8):859-65. doi: 10.1016/s0041-624x(02)00227-5. Ultrasonics. 2002. PMID: 12160059 Review.
Cited by
-
Estimating nonlinear anisotropic properties of healthy and aneurysm ascending aortas using magnetic resonance imaging.Biomech Model Mechanobiol. 2025 Feb;24(1):233-250. doi: 10.1007/s10237-024-01907-6. Epub 2024 Nov 26. Biomech Model Mechanobiol. 2025. PMID: 39586942 Free PMC article.
-
Fully coupled hybrid in-silico modeling of atherosclerosis: A multi-scale framework integrating CFD, transport phenomena and agent-based modeling.Front Bioeng Biotechnol. 2025 Mar 28;13:1549104. doi: 10.3389/fbioe.2025.1549104. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40225118 Free PMC article.
-
FEBio FINESSE: An Open-Source Finite Element Simulation Approach to Estimate In Vivo Heart Valve Strains Using Shape Enforcement.Ann Biomed Eng. 2025 Jan;53(1):241-259. doi: 10.1007/s10439-024-03637-3. Epub 2024 Nov 5. Ann Biomed Eng. 2025. PMID: 39499365
References
-
- Akyildiz A. C., Hansen H. H., Nieuwstadt H. A., Speelman L., De Korte C. L., van der Steen A. F., et al. (2016). A framework for local mechanical characterization of atherosclerotic plaques: combination of ultrasound displacement imaging and inverse finite element analysis. Ann. Biomed. Eng. 44, 968–979. 10.1007/s10439-015-1410-8 - DOI - PMC - PubMed
-
- Avril S., Grédiac M., Pierron F. (2004). Sensitivity of the virtual fields method to noisy data. Comput. Mech. 34, 439–452. 10.1007/s00466-004-0589-6 - DOI
LinkOut - more resources
Full Text Sources

