Novel antibody competition binding assay identifies distinct serological profiles associated with protection
- PMID: 38152401
- PMCID: PMC10752609
- DOI: 10.3389/fimmu.2023.1303446
Novel antibody competition binding assay identifies distinct serological profiles associated with protection
Abstract
Introduction: Pre-erythrocytic malaria vaccines hold the promise of inducing sterile protection thereby preventing the morbidity and mortality associated with Plasmodium infection. The main surface antigen of P. falciparum sporozoites, i.e., the circumsporozoite protein (CSP), has been extensively explored as a target of such vaccines with significant success in recent years. Systematic adjuvant selection, refinements of the immunization regimen, and physical properties of the antigen may all contribute to the potential of increasing the efficacy of CSP-based vaccines. Protection appears to be dependent in large part on CSP antibodies. However due to a knowledge gap related to the exact correlates of immunity, there is a critical need to improve our ability to down select candidates preclinically before entering clinical trials including with controlled human malaria infections (CHMI).
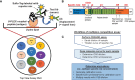
Methods: We developed a novel multiplex competition assay based on well-characterized monoclonal antibodies (mAbs) that target crucial epitopes across the CSP molecule. This new tool assesses both, quality and epitope-specific concentrations of vaccine-induced antibodies by measuring their equivalency with a panel of well-characterized, CSP-epitope-specific mAbs.
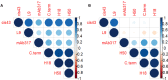
Results: Applying this method to RTS,S-immune sera from a CHMI trial demonstrated a quantitative epitope-specificity profile of antibody responses that can differentiate between protected vs. nonprotected individuals. Aligning vaccine efficacy with quantitation of the epitope fine specificity results of this equivalency assay reveals the importance of epitope specificity.
Discussion: The newly developed serological equivalence assay will inform future vaccine design and possibly even adjuvant selection. This methodology can be adapted to other antigens and disease models, when a panel of relevant mAbs exists, and could offer a unique tool for comparing and down-selecting vaccine formulations.
Keywords: antigen-specificity; circumsporozoite protein; equivalency; malaria; protection; serology; vaccine.
Copyright © 2023 Bolton, MacGill, Locke, Regules and Bergmann-Leitner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Kelleher K, Drysdale C. Who Recommends Groundbreaking Malaria Vaccine for Children at Risk (2019). World Health Organization. Available at: https://www.who.int/news/item/06-10-2021 (Accessed March 16, 2023).
-
- Cairns M, Barry A, Zongo I, Sagara I, Yerbanga SR, Diarra M, et al. . The duration of protection against clinical malaria provided by the combination of seasonal rts,S/as01(E) vaccination and seasonal malaria chemoprevention versus either intervention given alone. BMC Med (2022) 20(1):352. doi: 10.1186/s12916-022-02536-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

