Fixed low dose versus concentration-controlled initial tacrolimus dosing with reduced target levels in the course after kidney transplantation: results from a prospective randomized controlled non-inferiority trial (Slow & Low study)
- PMID: 38152417
- PMCID: PMC10751828
- DOI: 10.1016/j.eclinm.2023.102381
Fixed low dose versus concentration-controlled initial tacrolimus dosing with reduced target levels in the course after kidney transplantation: results from a prospective randomized controlled non-inferiority trial (Slow & Low study)
Abstract
Background: Optimal initial tacrolimus dosing and early exposure of tacrolimus after renal transplantation is not well studied.
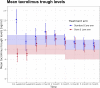
Methods: In this open-label, 6 months, multicenter, randomized controlled, non-inferiority study, we randomly assigned 432 renal allograft recipients to receive basiliximab induction, mycophenolate and steroids and either standard prolonged-release tacrolimus (trough levels: 7-9 ng/ml; Standard Care arm), or an initial 7-day fixed 5 mg/day dose of prolonged-release tacrolimus followed by lower tacrolimus predose levels (trough levels: 5-7 ng/ml; Slow & Low arm). The primary end point was the combined incidence rate of biopsy-proven acute rejections (BPAR; including borderline), graft failure, or death at 6 months with a non-inferiority margin of 12.5%. (EudraCT-Nr: 2013-001770-19.
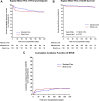
Findings: The combined primary endpoint in the Slow & Low arm was non-inferior compared to the Standard Care arm (22.1% versus 20.7%; difference: 1.4%, 90% CI -5.5% to 8.3%). The overall rate of BPAR including borderlines was similar (Slow & Low 17.4% versus Standard Care 16.6%). Safety parameters such as delayed graft function, kidney function, donor specific HLA-antibodies, infections, or post-transplantation diabetes mellitus did not differ.
Interpretation: This is the first study to show that an initial fixed dose of 5 mg per day followed by lower tacrolimus exposure is non-inferior compared to standard tacrolimus therapy and equally efficient and safe within 6 months after renal transplantation. These data suggest that therapeutic drug monitoring for prolonged release tacrolimus can be abandoned until start of the second week after transplantation.
Funding: Investigator-initiated trial, financial support by Astellas Pharma GmbH.
Keywords: Fixed low dose; Immunosuppression; Renal transplantation; Tacrolimus monitoring.
© 2023 The Author(s).
Conflict of interest statement
CH received grants from Astellas Pharma GmbH during the conduct of the study as well as grants and other from Novartis, other from Astellas, other from Chiesi, grants and other from AstraZeneca, grants and other from Hansa Biopharma, other from Alexion, grants and other from Boehringer Ingelheim, other from Takeda, other from Vifor Pharma, grants and other from Otsuka, other from Stadapharm, other from GlaxoSmithKline, other from Bayer Vital, other from MSD, grants from Calliditas, grants from Vera Therapeutics outside the submitted work. OW has received research grants for clinical studies, speaker's fees, honoraria and travel expenses from Amgen, Alexion, Astellas, Basilea, Biotest, Bristol-Myers Squibb, Correvio, Chiesi, Gilead, Hexal, Janssen, Dr. F. Köhler Chemie, MSD, Novartis, Roche, Pfizer, Sanofi, TEVA, AstraZeneca, GSK and UCB. KB received grants from Astellas during the conduct of the study; grants from Astellas, Chiesi, Alexion, CSL-Behring, MSD, personal fees from MSD, Takeda, Natera, Aicuris, CareDx, Stada, CSL-Behring, Paladin, Veloxis, Alexion, Pirche, Vifor, Carealytics, Neovii, outside the submitted work. TV received personal fees from Astellas Pharma GmbH outside the submitted work. ET received grants from University Hospital Carl Gustav Carus at the Technische Universität Dresden, Department of Internal Medicine III, Division of Nephrology during the conduct of the study. All other authors of this manuscript have no conflicts of interest to be disclosed.
Figures



References
-
- Ekberg H., Tedesco-Silva H., Demirbas A., et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. - PubMed
-
- Frei U., Daloze P., Vítko S., et al. Acute rejection in low-toxicity regimens: clinical impact and risk factors in the Symphony study. Clin Transplant. 2010;24(4):500–509. - PubMed
-
- Ekberg H., van Gelder T., Kaplan B., et al. Relationship of tacrolimus exposure and mycophenolate mofetil dose with renal function after renal transplantation. Transplantation. 2011;92(1):82–87. - PubMed
-
- Bouamar R., Shuker N., Hesselink D.A., et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials. Am J Transplant. 2013;13(5):1253–1261. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

