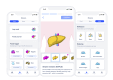
User Engagement, Acceptability, and Clinical Markers in a Digital Health Program for Nonalcoholic Fatty Liver Disease: Prospective, Single-Arm Feasibility Study
- PMID: 38152892
- PMCID: PMC10905363
- DOI: 10.2196/52576
User Engagement, Acceptability, and Clinical Markers in a Digital Health Program for Nonalcoholic Fatty Liver Disease: Prospective, Single-Arm Feasibility Study
Abstract
Background: Nonalcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease in the world. Common comorbidities are central obesity, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome. Cardiovascular disease is the most common cause of death among people with NAFLD, and lifestyle changes can improve health outcomes.
Objective: This study aims to explore the acceptability of a digital health program in terms of engagement, retention, and user satisfaction in addition to exploring changes in clinical outcomes, such as weight, cardiometabolic risk factors, and health-related quality of life.
Methods: We conducted a prospective, open-label, single-arm, 12-week study including 38 individuals with either a BMI >30, metabolic syndrome, or type 2 diabetes mellitus and NAFLD screened by FibroScan. An NAFLD-specific digital health program focused on disease education, lowering carbohydrates in the diet, food logging, increasing activity level, reducing stress, and healthy lifestyle coaching was offered to participants. The coach provided weekly feedback on food logs and other in-app activities and opportunities for participants to ask questions. The coaching was active throughout the 12-week intervention period. The primary outcome was feasibility and acceptability of the 12-week program, assessed through patient engagement, retention, and satisfaction with the program. Secondary outcomes included changes in weight, liver fat, body composition, and other cardiometabolic clinical parameters at baseline and 12 weeks.
Results: In total, 38 individuals were included in the study (median age 59.5, IQR 46.3-68.8 years; n=23, 61% female). Overall, 34 (89%) participants completed the program and 29 (76%) were active during the 12-week program period. The median satisfaction score was 6.3 (IQR 5.8-6.7) of 7. Mean weight loss was 3.5 (SD 3.7) kg (P<.001) or 3.2% (SD 3.4%), with a 2.2 (SD 2.7) kg reduction in fat mass (P<.001). Relative liver fat reduction was 19.4% (SD 23.9%). Systolic blood pressure was reduced by 6.0 (SD 13.5) mmHg (P=.009). The median reduction was 0.14 (IQR 0-0.47) mmol/L for triglyceride levels (P=.003), 3.2 (IQR 0.0-5.4) µU/ml for serum insulin (s-insulin) levels (P=.003), and 0.5 (IQR -0.7 to 3.8) mmol/mol for hemoglobin A1c (HbA1c) levels (P=.03). Participants who were highly engaged (ie, who used the app at least 5 days per week) had greater weight loss and liver fat reduction.
Conclusions: The 12-week-long digital health program was feasible for individuals with NAFLD, receiving high user engagement, retention, and satisfaction. Improved liver-specific and cardiometabolic health was observed, and more engaged participants showed greater improvements. This digital health program could provide a new tool to improve health outcomes in people with NAFLD.
Trial registration: Clinicaltrials.gov NCT05426382; https://clinicaltrials.gov/study/NCT05426382.
Keywords: BMI; NAFLD; acceptability; body composition; cardiology; cardiometabolic; cardiometabolic health; cardiovascular; chronic; coach; coaching; diabetes; diabetic; diabetics; diet; dietary; digital health; digital health program; digital therapeutics; exercise; fat; feasibility; hepatic; lifestyle; liver; metabolic syndrome; nonalcoholic fatty liver disease; nutrition; nutritional; patient education; physical activity; type 2; weight.
©Sigridur Björnsdottir, Hildigunnur Ulfsdottir, Elias Freyr Gudmundsson, Kolbrun Sveinsdottir, Ari Pall Isberg, Bartosz Dobies, Gudlaug Erla Akerlie Magnusdottir, Thrudur Gunnarsdottir, Tekla Karlsdottir, Gudlaug Bjornsdottir, Sigurdur Sigurdsson, Saemundur Oddsson, Vilmundur Gudnason. Originally published in JMIR Cardio (https://cardio.jmir.org), 15.02.2024.
Conflict of interest statement
Conflicts of Interest: HU, EFG, AI, BD, KS, TK, GEAM, and TG are employed by Sidekick Health. SO is an employee and cofounder of Sidekick Health. SB received consultancy fees from Sidekick Health during the study period. SS, GB, and VG have no competing interests to declare.
Figures
Similar articles
-
Engagement, Retention, and Acceptability in a Digital Health Program for Atopic Dermatitis: Prospective Interventional Study.JMIR Form Res. 2023 Jun 14;7:e41227. doi: 10.2196/41227. JMIR Form Res. 2023. PMID: 36975050 Free PMC article.
-
Evaluating the Feasibility of a Digital Therapeutic Program for Patients With Cancer During Active Treatment: Pre-Post Interventional Study.JMIR Form Res. 2022 Oct 13;6(10):e39764. doi: 10.2196/39764. JMIR Form Res. 2022. PMID: 36227639 Free PMC article.
-
Effect of Smartphone-Based Lifestyle Coaching App on Community-Dwelling Population With Moderate Metabolic Abnormalities: Randomized Controlled Trial.J Med Internet Res. 2020 Oct 9;22(10):e17435. doi: 10.2196/17435. J Med Internet Res. 2020. PMID: 33034564 Free PMC article. Clinical Trial.
-
Nonalcoholic Fatty Liver Disease: An Emerging Modern-Day Risk Factor for Cardiovascular Disease.Cureus. 2022 May 30;14(5):e25495. doi: 10.7759/cureus.25495. eCollection 2022 May. Cureus. 2022. PMID: 35783879 Free PMC article. Review.
-
Role of Nonalcoholic Fatty Liver Disease in Periodontitis: A Bidirectional Relationship.Cureus. 2024 Jul 3;16(7):e63775. doi: 10.7759/cureus.63775. eCollection 2024 Jul. Cureus. 2024. PMID: 39100036 Free PMC article. Review.
Cited by
-
Effects of exercise and a lifestyle app on sleep disordered breathing, physical health and quality of life.ERJ Open Res. 2025 Jun 23;11(3):01134-2024. doi: 10.1183/23120541.01134-2024. eCollection 2025 May. ERJ Open Res. 2025. PMID: 40551796 Free PMC article.
-
The Impact of Digital Technology-Based Exercise Combined With Dietary Intervention on Body Composition in College Students With Obesity: Prospective Study.J Med Internet Res. 2025 Jun 2;27:e65868. doi: 10.2196/65868. J Med Internet Res. 2025. PMID: 40455565 Free PMC article.
-
Feasibility of a digital lifestyle intervention (VITALISE) to support weight loss in patients with MASLD in routine secondary care.BMJ Open Gastroenterol. 2025 Jun 30;12(1):e001771. doi: 10.1136/bmjgast-2025-001771. BMJ Open Gastroenterol. 2025. PMID: 40588354 Free PMC article.
-
Effect of a digital health intervention on outpatients with heart failure: a randomized, controlled trial.Eur Heart J Digit Health. 2025 Jun 10;6(4):749-762. doi: 10.1093/ehjdh/ztaf063. eCollection 2025 Jul. Eur Heart J Digit Health. 2025. PMID: 40703118 Free PMC article.
References
-
- European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016 Jun;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004.S0168-8278(15)00734-5 - DOI - PubMed
-
- Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019 Oct;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021.S0168-8278(19)30393-9 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous