The disordered N-terminal tail of SARS-CoV-2 Nucleocapsid protein forms a dynamic complex with RNA
- PMID: 38153183
- PMCID: PMC10954482
- DOI: 10.1093/nar/gkad1215
The disordered N-terminal tail of SARS-CoV-2 Nucleocapsid protein forms a dynamic complex with RNA
Abstract
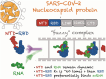
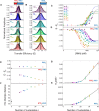
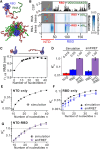
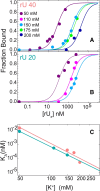
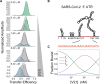
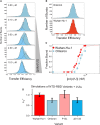
The SARS-CoV-2 Nucleocapsid (N) protein is responsible for condensation of the viral genome. Characterizing the mechanisms controlling nucleic acid binding is a key step in understanding how condensation is realized. Here, we focus on the role of the RNA binding domain (RBD) and its flanking disordered N-terminal domain (NTD) tail, using single-molecule Förster Resonance Energy Transfer and coarse-grained simulations. We quantified contact site size and binding affinity for nucleic acids and concomitant conformational changes occurring in the disordered region. We found that the disordered NTD increases the affinity of the RBD for RNA by about 50-fold. Binding of both nonspecific and specific RNA results in a modulation of the tail configurations, which respond in an RNA length-dependent manner. Not only does the disordered NTD increase affinity for RNA, but mutations that occur in the Omicron variant modulate the interactions, indicating a functional role of the disordered tail. Finally, we found that the NTD-RBD preferentially interacts with single-stranded RNA and that the resulting protein:RNA complexes are flexible and dynamic. We speculate that this mechanism of interaction enables the Nucleocapsid protein to search the viral genome for and bind to high-affinity motifs.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

