Race, Gene Expression Signatures, and Clinical Outcomes of Patients With High-Risk Early Breast Cancer
- PMID: 38153734
- PMCID: PMC10755617
- DOI: 10.1001/jamanetworkopen.2023.49646
Race, Gene Expression Signatures, and Clinical Outcomes of Patients With High-Risk Early Breast Cancer
Abstract
Importance: There has been little consideration of genomic risk of recurrence by breast cancer subtype despite evidence of racial disparities in breast cancer outcomes.
Objective: To evaluate associations between clinical trial end points, namely pathologic complete response (pCR) and distant recurrence-free survival (DRFS), and race and examine whether gene expression signatures are associated with outcomes by race.
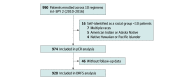
Design, setting, and participants: This retrospective cohort study used data from the Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging and Molecular Analysis 2 (I-SPY 2) multicenter clinical trial of neoadjuvant chemotherapy with novel agents and combinations for patients with previously untreated stage II/III breast cancer. Analyses were conducted of associations between race and short- and long-term outcomes, overall and by receptor subtypes, and their association with 28 expression biomarkers. The trial enrolled 990 female patients between March 30, 2010, and November 5, 2016, with a primary tumor size of 2.5 cm or greater and clinical or molecular high risk based on MammaPrint or hormone receptor (HR)-negative/ERBB2 (formerly HER2 or HER2/neu)-positive subtyping across 9 arms. This data analysis was performed between June 10, 2021, and October 20, 2022.
Exposure: Race, tumor receptor subtypes, and genomic biomarker expression of early breast cancer.
Main outcomes and measures: The primary outcomes were pCR and DRFS assessed by race, overall, and by tumor subtype using logistic regression and Cox proportional hazards regression models. The interaction between 28 expression biomarkers and race, considering pCR and DRFS overall and within subtypes, was also evaluated.
Results: The analytic sample included 974 participants (excluding 16 self-reporting as American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or multiple races due to small sample sizes), including 68 Asian (7%), 120 Black (12%), and 786 White (81%) patients. Median (range) age at diagnosis was 47 (25-71) years for Asian, 49 (25-77) for Black, and 49 (23-73) years for White patients. The pCR rates were 32% (n = 22) for Asian, 30% for Black (n = 36), and 32% for White (n = 255) patients (P = .87). Black patients with HR-positive/ERBB2-negative tumors not achieving pCR had significantly worse DRFS than their White counterparts (hazard ratio, 2.28; 95% CI, 1.24-4.21; P = .01), with 5-year DRFS rates of 55% (n = 32) and 77% (n = 247), respectively. Black patients with HR-positive/ERBB2-negative tumors, compared with White patients, had higher expression of an interferon signature (mean [SD], 0.39 [0.87] and -0.10 [0.99]; P = .007) and, compared with Asian patients, had a higher mitotic score (mean [SD], 0.07 [1.08] and -0.69 [1.06]; P = .01) and lower estrogen receptor/progesterone receptor signature (mean [SD], 0.31 [0.90] and 1.08 [0.95]; P = .008). A transforming growth factor β signature had a significant association with race relative to pCR and DRFS, with a higher signature associated with lower pCR and worse DRFS outcomes among Black patients only.
Conclusions and relevance: The findings show that women with early high-risk breast cancer who achieve pCR have similarly good outcomes regardless of race, but Black women with HR-positive/ERBB2-negative tumors without pCR may have worse DRFS than White women, highlighting the need to develop and test novel biomarker-informed therapies in diverse populations.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

