Merging Bambus[6]uril and Biotin[6]uril into an Enantiomerically Pure Monofunctionalized Hybrid Macrocycle
- PMID: 38153981
- PMCID: PMC10789090
- DOI: 10.1021/acs.orglett.3c03715
Merging Bambus[6]uril and Biotin[6]uril into an Enantiomerically Pure Monofunctionalized Hybrid Macrocycle
Abstract
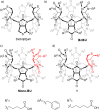
Bambus[6]urils and biotin[6]urils are macrocycles with an exceptional affinity for inorganic anions. Here, we investigated statistical condensation of 2,4-dibenzylglycoluril and d-biotin, monomers of the corresponding macrocycles, to prepare the enantiomerically pure macrocycle 1 containing a single d-biotin and five glycoluril units. Host-guest properties of 1 in chloroform solution and solid state were investigated. The macrocycle 1 bearing a single functional group was employed in the formation of [1]rotaxane utilizing reversible covalent bonds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Šindelář V.; Lízal T.. Chapter 20. Hemicucurbiturils. In Monographs in Supramolecular Chemistry; Kim K., Ed.; Royal Society of Chemistry: Cambridge, U.K., 2019; pp 527–545.10.1039/9781788015967-00527 - DOI
-
- Lizal T.; Sindelar V. Bambusuril Anion Receptors. Isr. J. Chem. 2018, 58 (3–4), 326–333. 10.1002/ijch.201700111. - DOI
-
- Lisbjerg M.; Pittelkow M.. Hemicucurbit[n]Urils. Comprehensive Supramolecular Chemistry II; Elsevier: 2017; pp 221–236.10.1016/B978-0-12-409547-2.12515-6 - DOI
LinkOut - more resources
Full Text Sources

