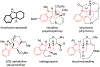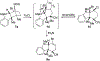Synthesis of Functionalized Hexahydrocarbazoles by Beckmann Elimination and Nucleophile-Intercepted Beckmann Fragmentation
- PMID: 38154135
- PMCID: PMC10843820
- DOI: 10.1021/acs.orglett.3c03434
Synthesis of Functionalized Hexahydrocarbazoles by Beckmann Elimination and Nucleophile-Intercepted Beckmann Fragmentation
Abstract
The Beckmann elimination and nucleophile-intercepted Beckmann fragmentation (NuBFr) of oximes starting from regioisomeric indolinyl bicyclic ketones lead to products that are subjected to further synthetic manipulations and ultimately result in the stereospecific formation of densely functionalized hexahydrocarbazoles. The Pd-catalyzed Suzuki-Miyaura cross-coupling reaction of a key alkenyl bromide intermediate with various boronic acids gives arylated products.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Selectivity Rules for the Dearomative (3+2) Annulation Reaction Between Substituted Indoles and Oxyallyl Cations.Adv Synth Catal. 2025 Apr 1;367(7):e202401536. doi: 10.1002/adsc.202401536. Epub 2025 Feb 26. Adv Synth Catal. 2025. PMID: 40276150
-
Diversification of Nucleophile-Intercepted Beckmann Fragmentation Products and Related Density Functional Theory Studies.J Org Chem. 2020 Sep 4;85(17):11396-11408. doi: 10.1021/acs.joc.0c01486. Epub 2020 Aug 26. J Org Chem. 2020. PMID: 32786611 Free PMC article.
-
Nucleophile-intercepted Beckmann fragmentation reactions.Chem Sci. 2019 Jul 4;10(33):7812-7815. doi: 10.1039/c9sc00926d. eCollection 2019 Sep 7. Chem Sci. 2019. PMID: 31588331 Free PMC article.
-
Suzuki-miyaura cross-coupling in acylation reactions, scope and recent developments.Molecules. 2013 Jan 17;18(1):1188-213. doi: 10.3390/molecules18011188. Molecules. 2013. PMID: 23344208 Free PMC article. Review.
-
Transmetalation of boronic acids and their derivatives: mechanistic elucidation and relevance to catalysis.Dalton Trans. 2022 Jan 17;51(3):777-796. doi: 10.1039/d1dt02986j. Dalton Trans. 2022. PMID: 34951434 Review.
Cited by
-
Rapid Access to Conjugated Z,Z,Z-Trienes.Angew Chem Int Ed Engl. 2025 May 26;64(22):e202502713. doi: 10.1002/anie.202502713. Epub 2025 Apr 21. Angew Chem Int Ed Engl. 2025. PMID: 40127016 Free PMC article.
-
Synthesis of Indolyl-7-azanorbornanes by Decomposition of Tetrahydro-1,2,3-triazepines.Adv Synth Catal. 2025 May 20;367(10):e202500047. doi: 10.1002/adsc.202500047. Epub 2025 Mar 17. Adv Synth Catal. 2025. PMID: 40486692
-
Selectivity Rules for the Dearomative (3+2) Annulation Reaction Between Substituted Indoles and Oxyallyl Cations.Adv Synth Catal. 2025 Apr 1;367(7):e202401536. doi: 10.1002/adsc.202401536. Epub 2025 Feb 26. Adv Synth Catal. 2025. PMID: 40276150
References
-
- Kay DP; Swindon P; Kennewell PD; Swindon O Hexahydrocarbazole Containing Alkylaminoamide Compounds, Pharmaceutical Compositions and Use. 4,782,075, November 1, 1988.
-
- Saxton JE Chapter 9: Synthesis of the Aspidosperma Alkaloids. In The Alkaloids: Chemistry and Biology; Elsevier, 1998; Vol. 50, pp 343–376. 10.1016/S1099-4831(08)60047-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources