Cell-Free and In Vivo Characterization of the Inhibitory Activity of Lavado Cocoa Flavanols on the Amyloid Protein Ataxin-3: Toward New Approaches against Spinocerebellar Ataxia Type 3
- PMID: 38154144
- PMCID: PMC10797631
- DOI: 10.1021/acschemneuro.3c00560
Cell-Free and In Vivo Characterization of the Inhibitory Activity of Lavado Cocoa Flavanols on the Amyloid Protein Ataxin-3: Toward New Approaches against Spinocerebellar Ataxia Type 3
Abstract
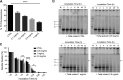
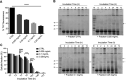
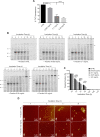
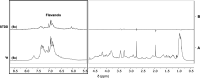
Spinocerebellar ataxia type 3 (SCA3) is a neurodegenerative disorder characterized by ataxia and other neurological manifestations, with a poor prognosis and a lack of effective therapies. The amyloid aggregation of the ataxin-3 protein is a hallmark of SCA3 and one of the main biochemical events prompting its onset, making it a prominent target for the development of preventive and therapeutic interventions. Here, we tested the efficacy of an aqueous Lavado cocoa extract and its polyphenolic components against ataxin-3 aggregation and neurotoxicity. The combination of biochemical assays and atomic force microscopy morphological analysis provided clear evidence of cocoa flavanols' ability to hinder ATX3 amyloid aggregation through direct physical interaction, as assessed by NMR spectroscopy. The chemical identity of the flavanols was investigated by ultraperformance liquid chromatography-high-resolution mass spectrometry. The use of the preclinical model Caenorhabditis elegans allowed us to demonstrate cocoa flavanols' ability to ameliorate ataxic phenotypes in vivo. To the best of our knowledge, Lavado cocoa is the first natural source whose extract is able to directly interfere with ATX3 aggregation, leading to the formation of off-pathway species.
Keywords: Caenorhabditis elegans; Lavado cocoa; NMR; UPLC-HR-MS; ataxin-3 protein (ATX3); flavanols; spinocerebellar ataxia type 3 (SCA3).
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Epigallocatechin-3-gallate and tetracycline differently affect ataxin-3 fibrillogenesis and reduce toxicity in spinocerebellar ataxia type 3 model.Hum Mol Genet. 2014 Dec 15;23(24):6542-52. doi: 10.1093/hmg/ddu373. Epub 2014 Jul 16. Hum Mol Genet. 2014. PMID: 25030034
-
Pathological ATX3 Expression Induces Cell Perturbations in E. coli as Revealed by Biochemical and Biophysical Investigations.Int J Mol Sci. 2021 Jan 19;22(2):943. doi: 10.3390/ijms22020943. Int J Mol Sci. 2021. PMID: 33477953 Free PMC article.
-
Targeting the VCP-binding motif of ataxin-3 improves phenotypes in Drosophila models of Spinocerebellar Ataxia Type 3.Neurobiol Dis. 2021 Dec;160:105516. doi: 10.1016/j.nbd.2021.105516. Epub 2021 Sep 24. Neurobiol Dis. 2021. PMID: 34563642 Free PMC article.
-
Toward therapeutic targets for SCA3: Insight into the role of Machado-Joseph disease protein ataxin-3 in misfolded proteins clearance.Prog Neurobiol. 2015 Sep;132:34-58. doi: 10.1016/j.pneurobio.2015.06.004. Epub 2015 Jun 27. Prog Neurobiol. 2015. PMID: 26123252 Review.
-
Autophagy in Spinocerebellar Ataxia Type 3: From Pathogenesis to Therapeutics.Int J Mol Sci. 2023 Apr 17;24(8):7405. doi: 10.3390/ijms24087405. Int J Mol Sci. 2023. PMID: 37108570 Free PMC article. Review.
Cited by
-
Dietary and lifestyle interventions for the management of hereditary ataxias.Front Nutr. 2025 Apr 24;12:1548821. doi: 10.3389/fnut.2025.1548821. eCollection 2025. Front Nutr. 2025. PMID: 40342369 Free PMC article. Review.
References
-
- Koeppen A. H.The neuropathology of spinocerebellar ataxia type 3/machado-joseph disease. In Polyglutamine Disorders; Nóbrega C., Pereira de Almeida L., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing, 2018; pp 233–241.10.1007/978-3-319-71779-1_11. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

