A real-world observational study assessing relationships between excessive daytime sleepiness and patient satisfaction in obstructive sleep apnea
- PMID: 38154148
- PMCID: PMC10961719
- DOI: 10.1016/j.sleep.2023.12.011
A real-world observational study assessing relationships between excessive daytime sleepiness and patient satisfaction in obstructive sleep apnea
Abstract
Objectives/background: To estimate prevalence and severity of excessive daytime sleepiness among patients with obstructive sleep apnea (OSA) who were prescribed treatment; assess perception and satisfaction of OSA-related care; describe relationships between excessive daytime sleepiness, treatment adherence, and patient satisfaction.
Patients/methods: A national population-based cross-sectional sample of US adults with clinician-diagnosed OSA was surveyed in January 2021 via Evidation Health's Achievement App. Patients completed the Epworth Sleepiness Scale, rated satisfaction with healthcare provider and overall OSA care, and reported treatment adherence. Covariates affecting excessive daytime sleepiness (average weekly sleep duration, treatment adherence, sleepiness-inducing medications, age, sex, body mass index, nasal congestion, smoking status, and comorbidities) were adjusted in multivariate regression models.
Results: In 2289 participants (50.3 % women; 44.8 ± 11.1 years), EDS was highly prevalent (42 %), and was experienced by 36 % of patients with high positive airway pressure (PAP) therapy adherence. Each additional hour of nightly PAP use was associated with improved sleepiness (a 0.28-point lower Epworth score; p < 0.001). Excessive daytime sleepiness was associated with lower patient satisfaction with healthcare providers and overall care (OR [95 % CI] 0.62 [0.48-0.80] and 0.50 [0.39-0.64], respectively; p < 0.0001), whereas PAP adherence was associated with higher patient satisfaction (OR [95 % CI] 2.37 [1.64-3.43] and 2.91 [2.03-4.17]; p < 0.0001), after adjusting for confounders.
Conclusions: In a real-world population-based study of patients with OSA, excessive daytime sleepiness was highly prevalent and associated with poor patient satisfaction ratings. Better patient-centered care among patients with OSA may require interventions aimed at addressing excessive daytime sleepiness and treatment adherence.
Keywords: Apnea; Excessive daytime sleepiness; Patient satisfaction; Treatment adherence.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest S Parthasarathy is a consultant for Jazz Pharmaceuticals, Inc., Abbvie, Inc., and Apria Healthcare. He reports receiving research grants to institution from Verily Lifesciences, Inc., Philips, Inc., Sommetrics, Inc., WHOOP, Inc., Regeneron, Inc., and USBiotest, Inc. Additionally, he reports receiving personal fees from UpToDate, Inc. Dr. Parthasarathy reports grants from NIH (R25-HL126140, R33-HL151254; OT2-HL161847; R21-HD109777; C06-OD028307; HL140144; HL138377; 1OT2HL156812; OT2-HL156912 and OT2HL158287), PCORI (DI-2018C2-13161, CER-2018C2-13262), Department of Defense (W81XWH20C0051 and W81XWH2110025), Pima County Health Department (CPIMP211275), Arizona Commerce Authority (LTR DTD 021822), and Sergey Brin Foundation. In addition, Dr. Parthasarathy has a patent US20160213879A1 licensed to SaiOx, Inc. that is unrelated to this manuscript. D Hyman, R Saad, S Morris, J Zhang, and G Parks are former employees of Jazz Pharmaceuticals who, in the course of this employment, received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. G Parks is currently a full-time employee of Axsome Therapeutics, Inc who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Axsome Therapeutics, Inc. J Doherty is an employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. L Eldemir, B Fox, MKY Vang, and J Schroeder, are former employees of Evidation, a consulting firm that received research funding from Jazz Pharmaceuticals to conduct this study. NJ Marshall is an employee of Evidation, a consulting firm that received research funding from Jazz Pharmaceuticals to conduct this study.
Figures
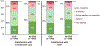
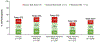

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

