Antimüllerian hormone level predicts ovulation in women with polycystic ovary syndrome treated with clomiphene and metformin
- PMID: 38154770
- PMCID: PMC10978249
- DOI: 10.1016/j.fertnstert.2023.12.031
Antimüllerian hormone level predicts ovulation in women with polycystic ovary syndrome treated with clomiphene and metformin
Abstract
Objective: To describe the serum anti-Müllerian hormone (AMH) concentrations in a large, well-phenotyped cohort of women with polycystic ovary syndrome (PCOS) and evaluate whether AMH predicts successful ovulation induction in women treated with clomiphene and metformin.
Design: Secondary analysis of randomized controlled trial.
Setting: Not applicable.
Patient(s): A total of 333 women with anovulatory infertility attributed to PCOS who participated in the double-blind randomized trial entitled the Pregnancy in Polycystic Ovary Syndrome I (PPCOS I) study (registration number, NCT00068861) who had serum samples from baseline laboratory testing available for further serum analysis were studied.
Intervention(s): Not applicable.
Main outcome measure(s): The association between the baseline AMH levels in each of the 3 treatment groups and ovulation, pregnancy, and live birth rates were assessed.
Result(s): A total of 322 individuals had a baseline AMH concentration available, of which the mean AMH was 11.7 ± 8.3 ng/mL (range 0.1-43.0 ng/mL). With each unit (1 ng/mL) increase in baseline AMH, the odds of ovulation decreased by 10% (odds ratio, 0.90; 95% confidence interval, 0.86-0.93); this effect did not differ by treatment group. Women with a high baseline AMH concentration (>8 ng/mL) were significantly less likely to ovulate compared with those with a normal baseline AMH concentration (<4 ng/mL) (odds ratio, 0.23; 95% confidence interval, 0.05-0.68). This remained statistically significant when controlling for confounders, including age, body mass index, time in study, and Homeostatic Model Assessment for Insulin Resistance score. Ovulation occurred even at very high AMH concentrations; there was no maximum level noted at which no ovulation events occurred. Baseline AMH concentration was not associated with pregnancy or live birth rates when controlling for confounders.
Conclusion(s): These AMH values in well-phenotyped individuals with PCOS add to the literature and will aid in identifying AMH criteria for the diagnosis of PCOS. In women with infertility and PCOS, a higher AMH concentration was associated with reduced odds of ovulation with ovulation induction with clomiphene, clomiphene + metformin, and metformin.
Clinical trial registration number: The original trial from which this analysis is derived was entitled "Pregnancy in Polycystic Ovary Syndrome: A 30 Week Double-Blind Randomized Trial of Clomiphene Citrate, Metformin XR, and Combined Clomiphene Citrate/Metformin XR For the Treatment of Infertility in Women With Polycystic Ovary Syndrome" and was registered on ClinicalTrials.gov as number NCT00068861. The URL for the trial is https://clinicaltrials.gov/study/NCT00068861. The first subject was enrolled in November 2002.
Keywords: AMH; PCOS; ovulation; ovulation induction.
Copyright © 2023 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A.S.K. reports that Ansh Labs provided funding for AMH/LH assays and Northwestern University Department of OB/GYN Biostatistical Support Grant and the Ralph Kazer Reproductive Endocrinology Fellowship Fund provided funding for statistical analyses and sample shipments for the submitted work. L.H. has nothing to disclose. P.S. has nothing to disclose. D.A.A. has nothing to disclose. A.K. has nothing to disclose. B.K. has nothing to disclose. R.S.L. reports funding from UL1 TR002014 National Center for Advancing Translational Sciences, 5 R01AT009484-02 Inositol Supplementation to Treat Polycystic Ovary Syndrome: A Double-Blind Dose Ranging RCT (INSUPP-P) NIH/NCCIH, 5 R01 HD091350-04 The COMET-PCOS Trial: Comparing the effects of Oral Contraceptive Pills vs. Metformin in the medical management of overweight/obese women with Polycystic Ovary Syndrome NIH/University of Pennsylvania, Guerbet USA Therapeutic Effect of Sonographic Hysterosalpingography: Oil vs. Water Based Media: The SHOW Pilot Trial, 5 R01 HD083323-04 Functional Analysis of PCOS Candidate Genes NIH/NICHD, 1 R01HD100630-01 AMH Signaling Pathway Variation in PCOS NIH, Hass Avocado Board A Multi-Site Randomized Clinical Trial to Compare Healthy Eating Recommendations for Six Months on Changes in Visceral Adiposity in Overweight/Obese Americans (Penn State Avocado Study), and 5 U10HD055925-10REV Data Coordinator Center for the RMN; consulting fees from Covis Pharma GmbH (2022), Novo Nordisk (12/2021), and Insudd (2020); honorarium from Honorary Professor, National Research Center for Assisted Reproductive Technology and Reproductive Genetics, Shandong University, Jinan, China, 2012–present, Member, Steering Committee. Eastern Siberia PCOS Epidemiology & Phenotype study, Scientific Center of Family Health and Human Reproduction, Irkutsk, Russian Federation 2016–present, Member, International Advisory Panel, Developing, disseminating and implementing a core outcome set for infertility, funded by the Royal Society of New Zealand Catalyst Fund and the University of Auckland, New Zealand, 2017–present, Advisor, A metabolomic approach to redefine diagnostic criteria and classification of polycystic ovary syndrome (PI: Professor Chi Chui Wang, Chinese University of Hong Kong), funded by the Research Fund, Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (there are no in-person meetings.); PCOS Challenge (patient support group) Member, Medical/Scientific Advisory Board, 2017–present, Endocrine Society: Member Investment Subcommittee, 2018–present; Associate Editor, Human Reproduction Update, 2017-2020, Associate Editor, Fertility and Sterility, 2009-2020, Editorial Editor, Fertility and Sterility, 2020–present, Associate Editor, Global Reproductive Health, 2017–present; Owner of 1000 shares of Biodesix (BDSX); NIH, ZHD1 DSR-K (LR) 1 Loan Repayment Program (annually, 2008–present), Special Emphasis Panel/Scientific Review Group 10 ZHD1 DSR-M (55): National Centers for Translational Research in Reproduction and Infertility (2020), and Miscellaneous, Karolinska Institute, Stockholm, Sweden (2020) outside the submitted work. C.E.B. has nothing to disclose.
Figures
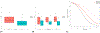
References
-
- ESHRE TR, Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and sterility 2004;81:19–25. - PubMed
-
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014;20:370–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HD038988/HD/NICHD NIH HHS/United States
- U10 HD039005/HD/NICHD NIH HHS/United States
- M01 RR010732/RR/NCRR NIH HHS/United States
- M01 RR000056/RR/NCRR NIH HHS/United States
- C06 RR016499/RR/NCRR NIH HHS/United States
- U10 HD038998/HD/NICHD NIH HHS/United States
- U10 HD038992/HD/NICHD NIH HHS/United States
- U10 HD038999/HD/NICHD NIH HHS/United States
- U10 HD033172/HD/NICHD NIH HHS/United States
- R01 HD029834/HD/NICHD NIH HHS/United States
- U10 HD027049/HD/NICHD NIH HHS/United States
- U01 HD038997/HD/NICHD NIH HHS/United States
- U10 HD027011/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

