Multimedia-based hormone therapy information program for patients with prostate cancer: the result of a randomized pilot study
- PMID: 38155164
- PMCID: PMC10754917
- DOI: 10.1038/s41598-023-50006-6
Multimedia-based hormone therapy information program for patients with prostate cancer: the result of a randomized pilot study
Abstract
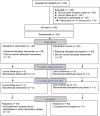
Few studies have explored the feasibility and efficacy of a multimedia information intervention for patients with prostate cancer who are undergoing hormone therapy. Thus, the purpose of the study was to assess the feasibility, acceptability, and the preliminary results of a multimedia-based hormone therapy information program (HTIP) on positive thinking and quality of life (QOL; primary outcomes) as well as social support and self-efficacy (secondary outcomes) of patients with prostate cancer. Patients with prostate cancer who were receiving hormone therapy were recruited from hospitals. After completing the pre-test questionnaire, patients were randomly divided into the multimedia information group (MIG; n = 40) and the control group (CG; n = 40). Patients in the MIG received a multimedia-based HTIP once a week for 6 weeks. Data were collected at 8 and 12 weeks after the pre-test. Measurement variables included positive thinking, QOL, social support, self-efficacy, and satisfaction with the program. The recruitment rate and retention rate were calculated for assessment of feasibility. The study had a 96.3% retention rate, and patients in the MIG were satisfied with the program. Preliminary results showed that, compared with those in the CG, patients in the MIG tended to exhibit higher positive thinking, prostate cancer-specific QOL, and social support at 8 weeks and 12 weeks after pre-test; however, the effect did not reach a statistically significant level. A multimedia-based HTIP is considered feasible and acceptable in patients with prostate cancer who underwent hormone therapy. Further research with a larger sample size, patients with high homogeneity in early-stage disease and long-term follow-up is needed to assess the efficacy of the intervention program.Trial registration: ClinicalTrials.gov (NCT04693910); Registered 05/01/2021.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
A Multimedia Self-management Intervention to Prepare Cancer Patients and Family Caregivers for Lung Surgery and Postoperative Recovery.Clin Lung Cancer. 2017 May;18(3):e151-e159. doi: 10.1016/j.cllc.2017.01.010. Epub 2017 Feb 2. Clin Lung Cancer. 2017. PMID: 28233696 Free PMC article.
-
Acceptability and preliminary feasibility of an internet/CD-ROM-based education and decision program for early-stage prostate cancer patients: randomized pilot study.J Med Internet Res. 2012 Jan 13;14(1):e6. doi: 10.2196/jmir.1891. J Med Internet Res. 2012. PMID: 22246148 Free PMC article. Clinical Trial.
-
Effectiveness of a couple-based psychosocial intervention on patients with prostate cancer and their partners: A quasi-experimental study.J Adv Nurs. 2020 Oct;76(10):2572-2585. doi: 10.1111/jan.14471. Epub 2020 Aug 3. J Adv Nurs. 2020. PMID: 32744426
-
The effectiveness and acceptability of multimedia information when recruiting children and young people to trials: the TRECA meta-analysis of SWATs.Health Soc Care Deliv Res. 2023 Nov;11(24):1-112. doi: 10.3310/HTPM3841. Health Soc Care Deliv Res. 2023. PMID: 38140894
-
Health-related quality of life, satisfaction, and economic outcome measures in studies of prostate cancer screening and treatment, 1990-2000.J Natl Cancer Inst Monogr. 2004;(33):78-101. doi: 10.1093/jncimonographs/lgh016. J Natl Cancer Inst Monogr. 2004. PMID: 15504921 Review.
Cited by
-
Effects on Quality of Life and Self-Efficacy of Instant Messaging Services in Self-Management Programs for Prostate Cancer: A Systematic Review and Meta-Analysis.Cancers (Basel). 2025 Jan 29;17(3):465. doi: 10.3390/cancers17030465. Cancers (Basel). 2025. PMID: 39941832 Free PMC article. Review.
References
-
- Eggener S. Hormonal therapy for prostate cancer. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, editors. Campbell-Walsh-Wein Urology. 12. Elsevier; 2021. pp. 3671–3686.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials