Fetal brain response to worsening acidosis: an experimental study in a fetal sheep model of umbilical cord occlusions
- PMID: 38155199
- PMCID: PMC10754920
- DOI: 10.1038/s41598-023-49495-2
Fetal brain response to worsening acidosis: an experimental study in a fetal sheep model of umbilical cord occlusions
Abstract
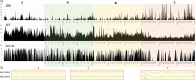
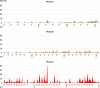
Perinatal anoxia remains an important public health problem as it can lead to hypoxic-ischaemic encephalopathy (HIE) and cause significant neonatal mortality and morbidity. The mechanisms of the fetal brain's response to hypoxia are still unclear and current methods of in utero HIE prediction are not reliable. In this study, we directly analysed the brain response to hypoxia in fetal sheep using in utero EEG. Near-term fetal sheep were subjected to progressive hypoxia induced by repeated umbilical cord occlusions (UCO) at increasing frequency. EEG changes during and between UCO were analysed visually and quantitatively, and related with gasometric and haemodynamic data. EEG signal was suppressed during occlusions and progressively slowed between occlusions with the increasing severity of the occlusions. Per-occlusion EEG suppression correlated with per-occlusion bradycardia and increased blood pressure, whereas EEG slowing and amplitude decreases correlated with arterial hypotension and respiratory acidosis. The suppression of the EEG signal during cord occlusion, in parallel with cardiovascular adaptation could correspond to a rapid cerebral adaptation mechanism that may have a neuroprotective role. The progressive alteration of the signal with the severity of the occlusions would rather reflect the cerebral hypoperfusion due to the failure of the cardiovascular adaptation mechanisms.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Fetal heart rate variability changes during brief repeated umbilical cord occlusion in near term fetal sheep.Br J Obstet Gynaecol. 1999 Jul;106(7):664-71. doi: 10.1111/j.1471-0528.1999.tb08365.x. Br J Obstet Gynaecol. 1999. PMID: 10428522
-
Dissecting the contributions of the peripheral chemoreflex and myocardial hypoxia to fetal heart rate decelerations in near-term fetal sheep.J Physiol. 2023 May;601(10):2017-2041. doi: 10.1113/JP284286. Epub 2023 Apr 19. J Physiol. 2023. PMID: 37017488
-
Brain Injury and Inflammatory Response to Umbilical Cord Occlusions Is Limited With Worsening Acidosis in the Near-Term Ovine Fetus.Reprod Sci. 2016 Jul;23(7):858-70. doi: 10.1177/1933719115623640. Epub 2015 Dec 23. Reprod Sci. 2016. PMID: 26704527
-
Tei index for prenatal diagnosis of acute fetal hypoxia due to intermittent umbilical cord occlusion in an animal model.Prenat Diagn. 2007 Sep;27(9):817-23. doi: 10.1002/pd.1781. Prenat Diagn. 2007. PMID: 17611944
-
Spontaneous hypoxia in multiple pregnancies is associated with early fetal decompensation and enhanced T-wave elevation during brief repeated cord occlusion in near-term fetal sheep.Am J Obstet Gynecol. 2005 Oct;193(4):1526-33. doi: 10.1016/j.ajog.2005.03.024. Am J Obstet Gynecol. 2005. PMID: 16202751
Cited by
-
The Importance of Including Maternal Immune Activation in Animal Models of Hypoxic-Ischemic Encephalopathy.Biomedicines. 2024 Nov 8;12(11):2559. doi: 10.3390/biomedicines12112559. Biomedicines. 2024. PMID: 39595123 Free PMC article.
References
-
- Garne E, Vain-Nielsen N, Hansen AV, Fenger-Grøn J. Birth asphyxia in a Danish hospital uptake area was reduced after centralisation of deliveries. Dan. Med. J. 2018;65(2):A5443. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

