Copper pyrithione and zinc pyrithione induce cytotoxicity and neurotoxicity in neuronal/astrocytic co-cultured cells via oxidative stress
- PMID: 38155222
- PMCID: PMC10754844
- DOI: 10.1038/s41598-023-49740-8
Copper pyrithione and zinc pyrithione induce cytotoxicity and neurotoxicity in neuronal/astrocytic co-cultured cells via oxidative stress
Abstract
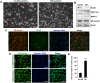
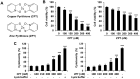
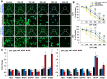
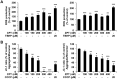
Previous studies on copper pyrithione (CPT) and zinc pyrithione (ZPT) as antifouling agents have mainly focused on marine organisms. Even though CPT and ZPT pose a risk of human exposure, their neurotoxic effects remain to be elucidated. Therefore, in this study, the cytotoxicity and neurotoxicity of CPT and ZPT were evaluated after the exposure of human SH-SY5Y/astrocytic co-cultured cells to them. The results showed that, in a co-culture model, CPT and ZPT induced cytotoxicity in a dose-dependent manner (~ 400 nM). Exposure to CPT and ZPT suppressed all parameters in the neurite outgrowth assays, including neurite length. In particular, exposure led to neurotoxicity at concentrations with low or no cytotoxicity (~ 200 nM). It also downregulated the expression of genes involved in neurodevelopment and maturation and upregulated astrocyte markers. Moreover, CPT and ZPT induced mitochondrial dysfunction and promoted the generation of reactive oxygen species. Notably, N-acetylcysteine treatment showed neuroprotective effects against CPT- and ZPT-mediated toxicity. We concluded that oxidative stress was the major mechanism underlying CPT- and ZPT-induced toxicity in the co-cultured cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Apoptosis in HepG2 cells induced by zinc pyrithione via mitochondrial dysfunction pathway: Involvement of zinc accumulation and oxidative stress.Ecotoxicol Environ Saf. 2018 Oct;161:515-525. doi: 10.1016/j.ecoenv.2018.06.026. Epub 2018 Jun 15. Ecotoxicol Environ Saf. 2018. PMID: 29913420
-
Seasonal variations in the effect of zinc pyrithione and copper pyrithione on pelagic phytoplankton communities.Aquat Toxicol. 2004 Aug 10;69(2):189-98. doi: 10.1016/j.aquatox.2004.05.006. Aquat Toxicol. 2004. PMID: 15261454
-
Indirect estimation of degradation time for zinc pyrithione and copper pyrithione in seawater.Mar Pollut Bull. 2004 May;48(9-10):894-901. doi: 10.1016/j.marpolbul.2003.11.013. Mar Pollut Bull. 2004. PMID: 15111036
-
Male Reproductive Toxicity of Antifouling Chemicals: Insights into Oxidative Stress-Induced Infertility and Molecular Mechanisms of Zinc Pyrithione (ZPT).Antioxidants (Basel). 2024 Jan 29;13(2):173. doi: 10.3390/antiox13020173. Antioxidants (Basel). 2024. PMID: 38397771 Free PMC article. Review.
-
Zinc Pyrithione: A Topical Antimicrobial With Complex Pharmaceutics.J Drugs Dermatol. 2016 Feb;15(2):140-4. J Drugs Dermatol. 2016. PMID: 26885780 Review.
Cited by
-
The Role of Autophagy in Copper-Induced Apoptosis and Developmental Neurotoxicity in SH-SY5Y Cells.Toxics. 2025 Jan 17;13(1):63. doi: 10.3390/toxics13010063. Toxics. 2025. PMID: 39853061 Free PMC article.
-
Pyrithione zinc alters mismatch repair to trigger tumor immunogenicity.Oncogene. 2025 Apr;44(14):983-995. doi: 10.1038/s41388-024-03272-1. Epub 2025 Jan 15. Oncogene. 2025. PMID: 39814851
-
Copper homeostasis and neurodegenerative diseases.Neural Regen Res. 2025 Nov 1;20(11):3124-3143. doi: 10.4103/NRR.NRR-D-24-00642. Epub 2024 Nov 13. Neural Regen Res. 2025. PMID: 39589160 Free PMC article.
-
Association of weekend catch-up sleep ratio with depressive risk: insights from NHANES 2021-2023.BMC Psychiatry. 2025 Jul 1;25(1):641. doi: 10.1186/s12888-025-07083-w. BMC Psychiatry. 2025. PMID: 40596991 Free PMC article.
References
-
- Schwartz JR. Zinc Pyrithione: A topical antimicrobial with complex pharmaceutics. J. Drugs Dermatol. 2016;15:140–144. - PubMed
-
- Union, E. Copper Pyrithione Product Type 21 88, (2015).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

