An in vivo drug screen in zebrafish reveals that cyclooxygenase 2-derived prostaglandin D2 promotes spinal cord neurogenesis
- PMID: 38155412
- PMCID: PMC11056714
- DOI: 10.1111/cpr.13594
An in vivo drug screen in zebrafish reveals that cyclooxygenase 2-derived prostaglandin D2 promotes spinal cord neurogenesis
Abstract
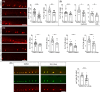
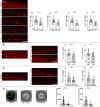
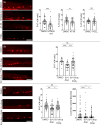
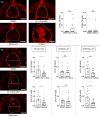
The study of neurogenesis is essential to understanding fundamental developmental processes and for the development of cell replacement therapies for central nervous system disorders. Here, we designed an in vivo drug screening protocol in developing zebrafish to find new molecules and signalling pathways regulating neurogenesis in the ventral spinal cord. This unbiased drug screen revealed that 4 cyclooxygenase (COX) inhibitors reduced the generation of serotonergic interneurons in the developing spinal cord. These results fitted very nicely with available single-cell RNAseq data revealing that floor plate cells show differential expression of 1 of the 2 COX2 zebrafish genes (ptgs2a). Indeed, several selective COX2 inhibitors and two different morpholinos against ptgs2a reduced the number of serotonergic neurons in the ventral spinal cord and led to locomotor deficits. Single-cell RNAseq data and different pharmacological manipulations further revealed that COX2-floor plate-derived prostaglandin D2 promotes neurogenesis in the developing spinal cord by promoting mitotic activity in progenitor cells. Rescue experiments using a phosphodiesterase-4 inhibitor suggest that intracellular changes in cAMP levels underlie the effects of COX inhibitors on neurogenesis and locomotion. Our study provides compelling in vivo evidence showing that prostaglandin signalling promotes neurogenesis in the ventral spinal cord.
© 2023 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Different Fgfs have distinct roles in regulating neurogenesis after spinal cord injury in zebrafish.Neural Dev. 2018 Nov 17;13(1):24. doi: 10.1186/s13064-018-0122-9. Neural Dev. 2018. PMID: 30447699 Free PMC article.
-
Insights into the mechanisms of neuron generation and specification in the zebrafish ventral spinal cord.FEBS J. 2024 Feb;291(4):646-662. doi: 10.1111/febs.16913. Epub 2023 Aug 3. FEBS J. 2024. PMID: 37498183 Review.
-
Dynamics of sonic hedgehog signaling in the ventral spinal cord are controlled by intrinsic changes in source cells requiring sulfatase 1.Development. 2014 Mar;141(6):1392-403. doi: 10.1242/dev.101717. Development. 2014. PMID: 24595292
-
A unique macrophage subpopulation signals directly to progenitor cells to promote regenerative neurogenesis in the zebrafish spinal cord.Dev Cell. 2021 Jun 7;56(11):1617-1630.e6. doi: 10.1016/j.devcel.2021.04.031. Epub 2021 May 24. Dev Cell. 2021. PMID: 34033756
-
The Temporal Mechanisms Guiding Interneuron Differentiation in the Spinal Cord.Int J Mol Sci. 2021 Jul 27;22(15):8025. doi: 10.3390/ijms22158025. Int J Mol Sci. 2021. PMID: 34360788 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

