Localisation of the centre of the highest region of muscle spindle abundance of anterior forearm muscles
- PMID: 38155435
- PMCID: PMC11021685
- DOI: 10.1111/joa.14000
Localisation of the centre of the highest region of muscle spindle abundance of anterior forearm muscles
Abstract
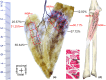
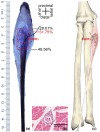
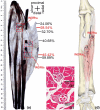
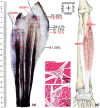
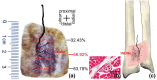
The centre of the highest region of muscle spindle abundance (CHRMSA) in the intramuscular nerve-dense region has been suggested as the optimal target location for injecting botulinum toxin A to block muscle spasms. The anterior forearm muscles have a high incidence of spasticity. However, the CHRMSA in the intramuscular nerve-dense region of the forearm anterior muscle group has not been defined. This study aimed to accurately define the body surface position and the depth of CHRMSA in an intramuscular nerve-dense region of the anterior forearm muscles. Twenty-four adult cadavers (57.7 ± 11.5 years) were included in this study. The curved line close to the skin connecting the medial and lateral epicondyles of the humerus was designated as the horizontal reference line (H line), and the line connecting the medial epicondyle of the humerus and the ulnar styloid was defined as the longitudinal reference line (L line). Modified Sihler's staining, haematoxylin-eosin staining and computed tomography scanning were employed to determine the projection points (P and P') of the CHRMSAs on the anterior and posterior surfaces of the forearm. The positions (PH and PL) of point P projected onto the H and L lines, and the depth of each CHRMSA, were determined using the Syngo system. The PH of the CHRMSA of the ulnar head of pronator teres, humeral head of pronator teres, flexor carpi radialis, palmaris longus, flexor carpi ulnaris, ulnar part of flexor digitorum superficialis, radial part of flexor digitorum superficialis, flexor pollicis longus, ulnar part of flexor digitorum profundus, radial portion of flexor digitorum profundus and pronator quadratus muscles were located at 42.48%, 45.52%, 41.20%, 19.70%, 7.77%, 25.65%, 47.42%, 53.47%, 12.28%, 38.41% and 51.68% of the H line, respectively; the PL were located at 18.38%, 12.54%, 28.83%, 13.43%, 17.65%, 32.76%, 57.32%, 64.12%, 20.05%, 45.94% and 88.71% of the L line, respectively; the puncture depths were located at 21.92%, 27.25%, 23.76%, 18.04%, 15.49%, 31.36%, 26.59%, 41.28%, 38.72%, 45.14% and 53.58% of the PP' line, respectively. The percentage values are the means of individual values. We recommend that the body surface puncture position and depth of the CHRMSA are the preferred locations for the intramuscular injection of botulinum toxin A to block anterior forearm muscle spasms.
Keywords: anterior forearm muscles; intramuscular nerve; muscle spindle abundance; spasm; target localization.
© 2023 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
None.
Figures




References
-
- Abo, M. , Shigematsu, T. , Hara, H. , Matsuda, Y. , Nimura, A. , Yamashita, Y. et al. (2020) Efficacy and safety of onabotulinumtoxina 400 units in patients with post‐stroke upper limb spasticity: final report of a randomized, double‐blind, placebo‐controlled trial with an open‐label extension phase. Toxins (Basel), 12(2), 127. - PMC - PubMed
-
- Akkaya, T. , Unlu, E. , Alptekin, A. , Gumus, H.I. , Umay, E. & Cakci, A. (2010) Neurolytic phenol blockade of the obturator nerve for severe adductor spasticity. Acta Anaesthesiologica Scandinavica, 54(1), 79–85. - PubMed
-
- Andresen, R. , Radmer, S. , Nickel, J. , Fischer, G. & Brinckmann, W. (2009) Ambulante CT‐gestützte thorakale Sympathikusblockade als zusätzliche Therapieoption bei komplexem regionalen Schmerzsyndrom Typ I nach Sportverletzungen [Ambulatory CT‐assisted thoracic sympathetic block as an additional approach to treatment of complex regional pain syndromes after sport injuries]. Sportverletzung Sportschaden, 23(1), 35–40. - PubMed
-
- Borodic, G.E. , Ferrante, R. , Pearce, L.B. & Smith, K. (1994) Histologic assessment of dose‐related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Movement Disorders, 9(1), 31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

