Phosphodiesterase inhibitor ameliorates senescent changes of renal interstitial pericytes in aging kidney
- PMID: 38155524
- PMCID: PMC10928568
- DOI: 10.1111/acel.14075
Phosphodiesterase inhibitor ameliorates senescent changes of renal interstitial pericytes in aging kidney
Abstract
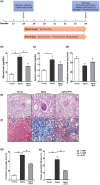
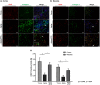
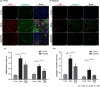
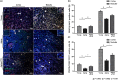
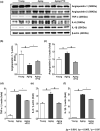
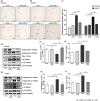
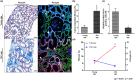
Pericytes are mesenchymal cells that surround endothelial cells, playing a crucial role in angiogenesis and vessel maturation. Additionally, they are associated with interstitial fibrosis as a major contributor to renal myofibroblasts. In this study, we aim to investigate whether the phosphodiesterase inhibitor, pentoxifylline (PTX), can ameliorate aging-related functional and histological deterioration in the kidney. We subjected aging C57BL/6 mice, dividing into young, aging, and PTX-treated aging groups. Renal function, albuminuria, and histological changes were assessed. Interstitial pericytes were assessed by immunohistochemistry analysis. We examined changes in pericytes in elderly patients using human kidney tissue obtained from healthy kidney donors for kidney transplantation. In vitro experiments with human pericytes and endothelial cells were performed. Aging mice exhibited declined renal function, increased albuminuria, and aging-related histological changes including mesangial expansion and tubulointerstitial fibrosis. Notably, number of pericytes declined in aging kidneys, and myofibroblasts increased. PTX treatment ameliorated albuminuria, histological alterations, and microvascular rarefaction, as well as modulated angiopoietin expression. In vitro experiments showed PTX reduced cellular senescence and inflammation. Human kidney analysis confirmed similar pericyte changes in aging kidneys. The phosphodiesterase inhibitor, PTX preserved microvascular density and improved renal interstitial fibrosis and inflammation in aging mice kidneys. These protective effects were suggested to be associated with the amelioration of pericytes reduction and the transition to myofibroblasts. Additionally, the upregulation of angiopoietin-1 expression may exert potential impacts. To the best of our knowledge, this is the first report on the changes in renal interstitial pericytes in aging human kidneys.
Keywords: aging; microvascular rarefaction; pentoxifylline; pericytes; phosphodiesterase inhibitors.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Berthiaume, A. A. , Schmid, F. , Stamenkovic, S. , Coelho‐Santos, V. , Nielson, C. D. , Weber, B. , Majesky, M. W. , & Shih, A. Y. (2022). Pericyte remodeling is deficient in the aged brain and contributes to impaired capillary flow and structure. Nature Communications, 13(1), 5912. 10.1038/s41467-022-33464-w - DOI - PMC - PubMed
-
- Chang, F. C. , Chiang, W. C. , Tsai, M. H. , Chou, Y. H. , Pan, S. Y. , Chang, Y. T. , Yeh, P. Y. , Chen, Y. T. , Chiang, C. K. , Chen, Y. M. , Chu, T. S. , Wu, K. D. , & Lin, S. L. (2014). Angiopoietin‐2‐induced arterial stiffness in CKD. Journal of the American Society of Nephrology: JASN, 25(6), 1198–1209. 10.1681/asn.2013050542 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

