Intracellular Application of an Asparaginyl Endopeptidase for Producing Recombinant Head-to-Tail Cyclic Proteins
- PMID: 38155637
- PMCID: PMC10751764
- DOI: 10.1021/jacsau.3c00591
Intracellular Application of an Asparaginyl Endopeptidase for Producing Recombinant Head-to-Tail Cyclic Proteins
Abstract
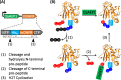
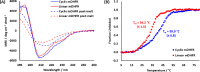
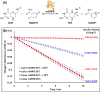
Peptide backbone cyclization is commonly observed in nature and is increasingly applied to proteins and peptides to improve thermal and chemical stability and resistance to proteolytic enzymes and enhance biological activity. However, chemical synthesis of head-to-tail cyclic peptides and proteins is challenging, is often low yielding, and employs toxic and unsustainable reagents. Plant derived asparaginyl endopeptidases such as OaAEP1 have been employed to catalyze the head-to-tail cyclization of peptides in vitro, offering a safer and more sustainable alternative to chemical methods. However, while asparaginyl endopeptidases have been used in vitro and in native and transgenic plant species, they have never been used to generate recombinant cyclic proteins in live recombinant organisms outside of plants. Using dihydrofolate reductase as a proof of concept, we show that a truncated OaAEP1 variant C247A is functional in the Escherichia coli physiological environment and can therefore be coexpressed with a substrate protein to enable concomitant in situ cyclization. The bacterial system is ideal for cyclic protein production owing to the fast growth rate, durability, ease of use, and low cost. This streamlines cyclic protein production via a biocatalytic process with fast kinetics and minimal ligation scarring, while negating the need to purify the enzyme, substrate, and reaction mixtures individually. The resulting cyclic protein was characterized in vitro, demonstrating enhanced thermal stability compared to the corresponding linear protein without impacting enzyme activity. We anticipate this convenient method for generating cyclic peptides will have broad utility in a range of biochemical and chemical applications.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Yeast-based bioproduction of disulfide-rich peptides and their cyclization via asparaginyl endopeptidases.Nat Protoc. 2021 Mar;16(3):1740-1760. doi: 10.1038/s41596-020-00483-0. Epub 2021 Feb 17. Nat Protoc. 2021. PMID: 33597770
-
Segmental isotopic labeling of a single-domain globular protein without any refolding step by an asparaginyl endopeptidase.FEBS Lett. 2017 May;591(9):1285-1294. doi: 10.1002/1873-3468.12640. Epub 2017 Apr 20. FEBS Lett. 2017. PMID: 28369872
-
In Vitro and In Planta Cyclization of Target Peptides Using an Asparaginyl Endopeptidase from Oldenlandia affinis.Methods Mol Biol. 2019;2012:211-235. doi: 10.1007/978-1-4939-9546-2_12. Methods Mol Biol. 2019. PMID: 31161511
-
Macrocyclization by asparaginyl endopeptidases.New Phytol. 2018 May;218(3):923-928. doi: 10.1111/nph.14511. Epub 2017 Mar 21. New Phytol. 2018. PMID: 28322452 Review.
-
Circular micro-proteins and mechanisms of cyclization.Curr Pharm Des. 2011 Dec;17(38):4318-28. doi: 10.2174/138161211798999410. Curr Pharm Des. 2011. PMID: 22204430 Review.
Cited by
-
Use of a head-to-tail peptide cyclase to prepare hybrid RiPPs.Chem Commun (Camb). 2024 Jun 20;60(51):6508-6511. doi: 10.1039/d3cc04919a. Chem Commun (Camb). 2024. PMID: 38833296 Free PMC article.
-
Genetic Code Expansion Approaches to Decipher the Ubiquitin Code.Chem Rev. 2024 Oct 23;124(20):11544-11584. doi: 10.1021/acs.chemrev.4c00375. Epub 2024 Sep 23. Chem Rev. 2024. PMID: 39311880 Free PMC article. Review.
-
Peptide and Protein Cyclization by a Promiscuous Graspetide Synthetase.ACS Cent Sci. 2025 Jun 9;11(7):1111-1121. doi: 10.1021/acscentsci.5c00408. eCollection 2025 Jul 23. ACS Cent Sci. 2025. PMID: 40726780 Free PMC article.
References
-
- Harris K. S.; Durek T.; Kaas Q.; Poth A. G.; Gilding E. K.; Conlan B. F.; Saska I.; Daly N. L.; van der Weerden N. L.; Craik D. J.; Anderson M. A. Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase. Nat. Commun. 2015, 6, 1019910.1038/ncomms10199. - DOI - PMC - PubMed
-
- Du J.; Yap K.; Chan L. Y.; Rehm F. B. H.; Looi F. Y.; Poth A. G.; Gilding E. K.; Kaas Q.; Durek T.; Craik D. J. A bifunctional asparaginyl endopeptidase efficiently catalyzes both cleavage and cyclization of cyclic trypsin inhibitors. Nat. Commun. 2020, 11 (1), 157510.1038/s41467-020-15418-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
