Designed Local Electric Fields-Promising Tools for Enzyme Engineering
- PMID: 38155642
- PMCID: PMC10752214
- DOI: 10.1021/jacsau.3c00536
Designed Local Electric Fields-Promising Tools for Enzyme Engineering
Abstract
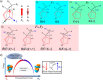
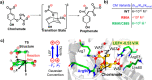
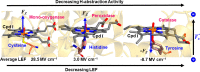
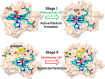
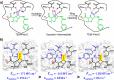
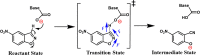
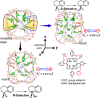
Designing efficient catalysts is one of the ultimate goals of chemists. In this Perspective, we discuss how local electric fields (LEFs) can be exploited to improve the catalytic performance of supramolecular catalysts, such as enzymes. More specifically, this Perspective starts by laying out the fundamentals of how local electric fields affect chemical reactivity and review the computational tools available to study electric fields in various settings. Subsequently, the advances made so far in optimizing enzymatic electric fields through targeted mutations are discussed critically and concisely. The Perspective ends with an outlook on some anticipated evolutions of the field in the near future. Among others, we offer some pointers on how the recent data science/machine learning revolution, engulfing all science disciplines, could potentially provide robust and principled tools to facilitate rapid inference of electric field effects, as well as the translation between optimal electrostatic environments and corresponding chemical modifications.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
