Dose-response technique combined with stable isotope tracing for drug metabolite profiling by using high-resolution mass spectrometry
- PMID: 38155901
- PMCID: PMC10753831
- DOI: 10.3389/fphar.2023.1293540
Dose-response technique combined with stable isotope tracing for drug metabolite profiling by using high-resolution mass spectrometry
Abstract
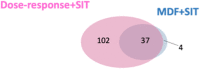
Background: Mass spectrometry metabolomics-based data-processing approaches have been developed for drug metabolite profiling. However, existing approaches cannot be used to comprehensively identify drug metabolites with high efficacy. Methods: Herein, we propose a two-stage data-processing approach for effective and comprehensive drug metabolite identification. The approach combines dose-response experiments with stable isotope tracing (SIT). Rosiglitazone (ROS), commonly used to treat type 2 diabetes, was employed as a model drug. Results: In the first stage of data processing, 1,071 features exhibited a dose-response relationship among 22,597 features investigated. In the second stage, these 1,071 features were screened for isotope pairs, and 200 features with isotope pairs were identified. In time-course experiments, a large proportion of the identified features (69.5%: 137 out of 200 features) were confirmed to be possible ROS metabolites. We compared the validated features identified using our approach with those identified using a previously reported approach [the mass defect filter (MDF) combined with SIT] and discovered that most of the validated features (37 out of 42) identified using the MDF-SIT combination were also successfully identified using our approach. Of the 143 validated features identified by both approaches, 74 had a proposed structure of an ROS-structure-related metabolite; the other 34 features that contained a specific fragment of ROS metabolites were considered possible ROS metabolites. Interestingly, numerous ROS-structure-related metabolites were identified in this study, most of which were novel. Conclusion: The results reveal that the proposed approach can effectively and comprehensively identify ROS metabolites.
Keywords: dose-response relationship; metabolomics; rosiglitazone; stable isotope tracing; time-course experiment.
Copyright © 2023 Lin, Chuang and Shih.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alvarez-Sánchez R., Montavon F., Hartung T., Pähler A. (2006). Thiazolidinedione bioactivation: a comparison of the bioactivation potentials of troglitazone, rosiglitazone, and pioglitazone using stable isotope-labeled analogues and liquid chromatography tandem mass spectrometry. Chem. Res. Toxicol. 19, 1106–1116. 10.1021/tx050353h - DOI - PubMed
-
- Anari M. R., Sanchez R. I., Bakhtiar R., Franklin R. B., Baillie T. A. (2004). Integration of knowledge-based metabolic predictions with liquid chromatography data-dependent tandem mass spectrometry for drug metabolism studies: application to studies on the biotransformation of indinavir. Anal. Chem. 76, 823–832. 10.1021/ac034980s - DOI - PubMed
LinkOut - more resources
Full Text Sources

