Cartilaginous endplates: A comprehensive review on a neglected structure in intervertebral disc research
- PMID: 38156054
- PMCID: PMC10751983
- DOI: 10.1002/jsp2.1294
Cartilaginous endplates: A comprehensive review on a neglected structure in intervertebral disc research
Abstract
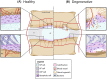
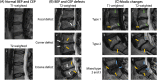
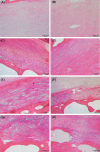
The cartilaginous endplates (CEP) are key components of the intervertebral disc (IVD) necessary for sustaining the nutrition of the disc while distributing mechanical loads and preventing the disc from bulging into the adjacent vertebral body. The size, shape, and composition of the CEP are essential in maintaining its function, and degeneration of the CEP is considered a contributor to early IVD degeneration. In addition, the CEP is implicated in Modic changes, which are often associated with low back pain. This review aims to tackle the current knowledge of the CEP regarding its structure, composition, permeability, and mechanical role in a healthy disc, how they change with degeneration, and how they connect to IVD degeneration and low back pain. Additionally, the authors suggest a standardized naming convention regarding the CEP and bony endplate and suggest avoiding the term vertebral endplate. Currently, there is limited data on the CEP itself as reported data is often a combination of CEP and bony endplate, or the CEP is considered as articular cartilage. However, it is clear the CEP is a unique tissue type that differs from articular cartilage, bony endplate, and other IVD tissues. Thus, future research should investigate the CEP separately to fully understand its role in healthy and degenerated IVDs. Further, most IVD regeneration therapies in development failed to address, or even considered the CEP, despite its key role in nutrition and mechanical stability within the IVD. Thus, the CEP should be considered and potentially targeted for future sustainable treatments.
Keywords: biologic therapies; biomechanics; degeneration; pre‐clinical models.
© 2023 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
Benjamin Gantenbein and Christine Le Maitre are editorial board members of JOR Spine and co‐author of this article. They were excluded from editorial decision‐making related to the acceptance of this article for publication in the journal. All other authors have no conflicts of interest to declare in relation to this article.
Figures

Similar articles
-
From structure to therapy: the critical influence of cartilaginous endplates and microvascular network on intervertebral disc degeneration.Front Bioeng Biotechnol. 2024 Oct 28;12:1489420. doi: 10.3389/fbioe.2024.1489420. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39530056 Free PMC article. Review.
-
Ultra-short echo time MR imaging in assessing cartilage endplate damage and relationship between its lesion and disc degeneration for chronic low back pain patients.BMC Med Imaging. 2023 Apr 20;23(1):60. doi: 10.1186/s12880-023-01014-5. BMC Med Imaging. 2023. PMID: 37081427 Free PMC article.
-
Insights into the mechanical microenvironment within the cartilaginous endplate: An emerging role in maintaining disc homeostasis and normal function.Heliyon. 2024 May 11;10(10):e31162. doi: 10.1016/j.heliyon.2024.e31162. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803964 Free PMC article. Review.
-
Comparing contribution of bony and cartilaginous endplate changes to intervertebral disc degeneration.Pak J Med Sci. 2024 Aug;40(7):1516-1522. doi: 10.12669/pjms.40.7.8762. Pak J Med Sci. 2024. PMID: 39092047 Free PMC article.
-
Transcriptional profiling of human cartilage endplate cells identifies novel genes and cell clusters underlying degenerated and non-degenerated phenotypes.Arthritis Res Ther. 2024 Jan 3;26(1):12. doi: 10.1186/s13075-023-03220-6. Arthritis Res Ther. 2024. PMID: 38173036 Free PMC article.
Cited by
-
Qualitative and Quantitative MR Imaging of the Cartilaginous Endplate: A Review.J Magn Reson Imaging. 2025 Apr;61(4):1552-1571. doi: 10.1002/jmri.29562. Epub 2024 Aug 20. J Magn Reson Imaging. 2025. PMID: 39165086 Review.
-
Pyroptosis: candidate key targets for mesenchymal stem cell-derived exosomes for the treatment of bone-related diseases.Stem Cell Res Ther. 2025 Feb 12;16(1):68. doi: 10.1186/s13287-025-04167-y. Stem Cell Res Ther. 2025. PMID: 39940049 Free PMC article. Review.
-
Advancing basic and preclinical spine research: Highlights from the ORS PSRS 6th International Spine Research Symposium.JOR Spine. 2023 Nov 21;6(4):e1308. doi: 10.1002/jsp2.1308. eCollection 2023 Dec. JOR Spine. 2023. PMID: 38156060 Free PMC article.
-
Sulfated Hydrogels as Primary Intervertebral Disc Cell Culture Systems.Gels. 2024 May 14;10(5):330. doi: 10.3390/gels10050330. Gels. 2024. PMID: 38786247 Free PMC article.
-
REDOX Imbalance and Oxidative Stress in the Intervertebral Disc: The Effect of Mechanical Stress and Cigarette Smoking on ER Stress and Mitochondrial Dysfunction.Cells. 2025 Apr 19;14(8):613. doi: 10.3390/cells14080613. Cells. 2025. PMID: 40277939 Free PMC article. Review.
References
-
- Lama P, Zehra U, Balkovec C, et al. Significance of cartilage endplate within herniated disc tissue. Eur Spine J. 2014;23:1869‐1877. - PubMed
-
- Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end‐plate and its relation to the intervertebral disc. Spine. 1989;14:166‐174. - PubMed
-
- Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700‐2709. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

