How to study biofilms: technological advancements in clinical biofilm research
- PMID: 38156318
- PMCID: PMC10753778
- DOI: 10.3389/fcimb.2023.1335389
How to study biofilms: technological advancements in clinical biofilm research
Abstract
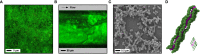
Biofilm formation is an important survival strategy commonly used by bacteria and fungi, which are embedded in a protective extracellular matrix of organic polymers. They are ubiquitous in nature, including humans and other animals, and they can be surface- and non-surface-associated, making them capable of growing in and on many different parts of the body. Biofilms are also complex, forming polymicrobial communities that are difficult to eradicate due to their unique growth dynamics, and clinical infections associated with biofilms are a huge burden in the healthcare setting, as they are often difficult to diagnose and to treat. Our understanding of biofilm formation and development is a fast-paced and important research focus. This review aims to describe the advancements in clinical biofilm research, including both in vitro and in vivo biofilm models, imaging techniques and techniques to analyse the biological functions of the biofilm.
Keywords: biofilm; biofilm analysis; biofilm imaging; biofilm model; host-microbe interactions; infection.
Copyright © 2023 Cleaver and Garnett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

