Mitofusin-2 Regulates Platelet Mitochondria and Function
- PMID: 38156445
- PMCID: PMC10872864
- DOI: 10.1161/CIRCRESAHA.123.322914
Mitofusin-2 Regulates Platelet Mitochondria and Function
Abstract
Background: Single-nucleotide polymorphisms linked with the rs1474868 T allele (MFN2 [mitofusin-2] T/T) in the human mitochondrial fusion protein MFN2 gene are associated with reduced platelet MFN2 RNA expression and platelet counts. This study investigates the impact of MFN2 on megakaryocyte and platelet biology.
Methods: Mice with megakaryocyte/platelet deletion of Mfn2 (Mfn2-/- [Mfn2 conditional knockout]) were generated using Pf4-Cre crossed with floxed Mfn2 mice. Human megakaryocytes were generated from cord blood and platelets isolated from healthy subjects genotyped for rs1474868. Ex vivo approaches assessed mitochondrial morphology, function, and platelet activation responses. In vivo measurements included endogenous/transfused platelet life span, tail bleed time, transient middle cerebral artery occlusion, and pulmonary vascular permeability/hemorrhage following lipopolysaccharide-induced acute lung injury.
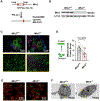
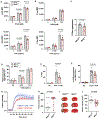
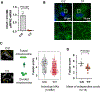
Results: Mitochondria was more fragmented in megakaryocytes derived from Mfn2-/- mice and from human cord blood with MFN2 T/T genotype compared with control megakaryocytes. Human resting platelets of MFN2 T/T genotype had reduced MFN2 protein, diminished mitochondrial membrane potential, and an increased rate of phosphatidylserine exposure during ex vivo culture. Platelet counts and platelet life span were reduced in Mfn2-/- mice accompanied by an increased rate of phosphatidylserine exposure in resting platelets, especially aged platelets, during ex vivo culture. Mfn2-/- also decreased platelet mitochondrial membrane potential (basal) and activated mitochondrial oxygen consumption rate, reactive oxygen species generation, calcium flux, platelet-neutrophil aggregate formation, and phosphatidylserine exposure following dual agonist activation. Ultimately, Mfn2-/- mice showed prolonged tail bleed times, decreased ischemic stroke infarct size after cerebral ischemia-reperfusion, and exacerbated pulmonary inflammatory hemorrhage following lipopolysaccharide-induced acute lung injury. Analysis of MFN2 SNPs in the iSPAAR study (Identification of SNPs Predisposing to Altered ALI Risk) identified a significant association between MFN2 and 28-day mortality in patients with acute respiratory distress syndrome.
Conclusions: Mfn2 preserves mitochondrial phenotypes in megakaryocytes and platelets and influences platelet life span, function, and outcomes of stroke and lung injury.
Keywords: blood platelets; ischemic stroke; megakaryocytes; mitochondria; neutrophils.
Conflict of interest statement
Figures




Comment in
-
Platelet Mitochondrial Fusion and Function in Vascular Integrity.Circ Res. 2024 Jan 19;134(2):162-164. doi: 10.1161/CIRCRESAHA.123.323867. Epub 2024 Jan 18. Circ Res. 2024. PMID: 38236952 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

