Bridge Cross-Coupling of Bicyclo[1.1.0]butanes
- PMID: 38156902
- PMCID: PMC10789093
- DOI: 10.1021/acs.orglett.3c04030
Bridge Cross-Coupling of Bicyclo[1.1.0]butanes
Abstract
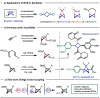
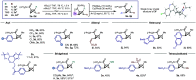
Bicyclo[1.1.0]butanes (BCBs) have gained growing popularity in "strain release" chemistry for the synthesis of four-membered-ring systems and para- and meta-disubstituted arene bioisosteres as well as applications in chemoselective bioconjugation. However, functionalization of the bridge position of BCBs can be challenging due to the inherent strain of the ring system and reactivity of the central C-C bond. Here we report the first late-stage bridge cross-coupling of BCBs, mediated by directed metalation/palladium catalysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
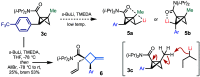
References
-
- Golfmann M.; Walker J. C. L. Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis. Commun. Chem. 2023, 6, 9.10.1038/s42004-022-00811-3. - DOI - PMC - PubMed
- Kelly C. B.; Milligan J. A.; Tilley L. J.; Sodano T. M. Bicyclobutanes: from curiosities to versatile reagents and covalent warheads. Chem. Sci. 2022, 13, 11721–11737. 10.1039/D2SC03948F. - DOI - PMC - PubMed
- Fawcett A. Recent advances in the chemistry of bicyclo- and 1-azabicyclo[1.1.0]butanes. Pure Appl. Chem. 2020, 92, 751–765. 10.1515/pac-2019-1007. - DOI
- Turkowska J.; Durka J.; Gryko D. Strain release – an old tool for new transformations. Chem. Commun. 2020, 56, 5718–5734. 10.1039/D0CC01771J. - DOI - PubMed
-
- Guo L.; Noble A.; Aggarwal V. K. α-Selective Ring-Opening Reactions of Bicyclo[1.1.0]butyl Boronic Ester with Nucleophiles. Angew. Chem., Int. Ed. 2021, 60, 212–216. 10.1002/anie.202011739. - DOI - PubMed
- Lopchuk J. M.; Fjelbye K.; Kawamata Y.; Malins L. R.; Pan C.-M.; Gianatassio R.; Wang J.; Prieto L.; Bradow J.; Brandt T. A.; Collins M. R.; Elleraas J.; Ewanicki J.; Farrell W.; Fadeyi O. O.; Gallego G. M.; Mousseau J. J.; Oliver R.; Sach N. W.; Smith J. K.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity. J. Am. Chem. Soc. 2017, 139, 3209–3226. 10.1021/jacs.6b13229. - DOI - PMC - PubMed
- Gianatassio R.; Lopchuk J. M.; Wang J.; Pan C.; Malins L. R.; Prieto L.; Brandt T. A.; Collins M. R.; Gallego G. M.; Sach N. W.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-release amination. Science 2016, 351, 241.10.1126/science.aad6252. - DOI - PMC - PubMed
- Panish R. A.; Chintala S. R.; Fox J. M. A Mixed-Ligand Chiral Rhodium(II) Catalyst Enables the Enantioselective Total Synthesis of Piperarborenine B. Angew. Chem., Int. Ed. 2016, 55, 4983–4987. 10.1002/anie.201600766. - DOI - PMC - PubMed
- Panish R.; Chintala S. R.; Boruta D. T.; Fang Y.; Taylor M. T.; Fox J. M. Enantioselective Synthesis of Cyclobutanes via Sequential Rh-catalyzed Bicyclobutanation/Cu-catalyzed Homoconjugate Addition. J. Am. Chem. Soc. 2013, 135, 9283–9286. 10.1021/ja403811t. - DOI - PMC - PubMed
- Gaoni Y. New bridgehead-substituted 1-(arylsulfonyl)bicyclo[1.1.0]butanes and some novel addition reactions of the bicyclic system. Tetrahedron 1989, 45, 2819–2840. 10.1016/S0040-4020(01)80112-5. - DOI
- Gaoni Y.; Tomazic A. Bridgehead reactivity, nucleophilic and radical additions, and lithium aluminum hydride reduction of 1-(arylsulfonyl)bicyclobutanes: general access to substituted, functionalized cyclobutanes. Syntheses of (±)-citrilol acetate, (±)-junionone, and the tricyclo[3.3.0.01,4]octane and tricyclo[4.3.0.01,7]nonane ring systems. J. Org. Chem. 1985, 50, 2948–2957. 10.1021/jo00216a028. - DOI
-
- Pratt C. J.; Aycock R. A.; King M. D.; Jui N. T. Radical α-C–H Cyclobutylation of Aniline Derivatives. Synlett 2020, 31, 51–54. 10.1055/s-0039-1690197. - DOI - PMC - PubMed
- Silvi M.; Aggarwal V. K. Radical Addition to Strained σ-Bonds Enables the Stereocontrolled Synthesis of Cyclobutyl Boronic Esters. J. Am. Chem. Soc. 2019, 141, 9511–9515. 10.1021/jacs.9b03653. - DOI - PubMed
-
- Guin A.; Bhattacharjee S.; Harariya M. S.; Biju A. T. Lewis acid-catalyzed diastereoselective carbofunctionalization of bicyclobutanes employing naphthols. Chem. Sci. 2023, 14, 6585–6591. 10.1039/D3SC01373A. - DOI - PMC - PubMed
- Kerner M. J.; Wipf P. Semipinacol-Type Rearrangements of [3-(Arylsulfonyl)bicyclo[1.1.0]butan-1-yl]alkanols. Org. Lett. 2021, 23, 3615–3619. 10.1021/acs.orglett.1c01004. - DOI - PubMed
- Bennett S. H.; Fawcett A.; Denton E. H.; Biberger T.; Fasano V.; Winter N.; Aggarwal V. K. Difunctionalization of C–C σ-Bonds Enabled by the Reaction of Bicyclo[1.1.0]butyl Boronate Complexes with Electrophiles: Reaction Development, Scope, and Stereochemical Origins. J. Am. Chem. Soc. 2020, 142, 16766–16775. 10.1021/jacs.0c07357. - DOI - PubMed
-
- Wölfl B.; Winter N.; Li J.; Noble A.; Aggarwal V. K. Strain-Release Driven Epoxidation and Aziridination of Bicyclo[1.1.0]butanes via Palladium Catalyzed σ-Bond Nucleopalladation. Angew. Chem., Int. Ed. 2023, 62, e20221706410.1002/anie.202217064. - DOI - PMC - PubMed
- Zhang Z.; Gevorgyan V. Palladium Hydride-Enabled Hydroalkenylation of Strained Molecules. J. Am. Chem. Soc. 2022, 144, 20875–20883. 10.1021/jacs.2c09045. - DOI - PMC - PubMed
- Pinkert T.; Das M.; Schrader M. L.; Glorius F. Use of Strain-Release for the Diastereoselective Construction of Quaternary Carbon Centers. J. Am. Chem. Soc. 2021, 143, 7648–7654. 10.1021/jacs.1c03492. - DOI - PubMed
- Ociepa M.; Wierzba A. J.; Turkowska J.; Gryko D. Polarity-Reversal Strategy for the Functionalization of Electrophilic Strained Molecules via Light-Driven Cobalt Catalysis. J. Am. Chem. Soc. 2020, 142, 5355–5361. 10.1021/jacs.0c00245. - DOI - PubMed
- Fawcett A.; Biberger T.; Aggarwal V. K. Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem. 2019, 11, 117–122. 10.1038/s41557-018-0181-x. - DOI - PubMed
- Walczak M. A. A.; Krainz T.; Wipf P. Ring-Strain-Enabled Reaction Discovery: New Heterocycles from Bicyclo[1.1.0]butanes. Acc. Chem. Res. 2015, 48, 1149–1158. 10.1021/ar500437h. - DOI - PubMed
- Walczak M. A. A.; Wipf P. Rhodium(I)-Catalyzed Cycloisomerizations of Bicyclobutanes. J. Am. Chem. Soc. 2008, 130, 6924–6925. 10.1021/ja802906k. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

