Season affects the estrogen system and the immune response of common carp
- PMID: 38157099
- PMCID: PMC11021253
- DOI: 10.1007/s10695-023-01286-2
Season affects the estrogen system and the immune response of common carp
Abstract
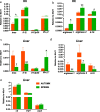
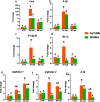
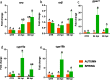
The physiology of ectothermic animals, including fish, is strictly regulated by season-related external factors such as temperature or photoperiod. The immune response and the production of hormones, such as estrogens, are therefore also subject to seasonal changes. This study in common carp aimed to determine how the season affects the estrogen system and the immune response, including the antibacterial response during Aeromonas salmonicida infection. We compared the immune reaction in spring and autumn in the head kidney and liver and found that carp have higher levels of blood 17β-estradiol in autumn, while in the liver of these fish there is a higher constitutive expression of genes encoding vitellogenin, estrogen receptors and Cyp19 aromatase than in spring. Fish sampled in autumn also exhibited higher expression of immune-related genes in the liver. In contrast, in the head kidney from fish sampled in the autumn, the expression of genes encoding estrogen receptors and aromatase was lower than in spring, and a similar profile of expression was also measured in the head kidney for inos, arginases and il-10. In turn, during bacterial infection, we observed higher upregulation of the expression of inos, il-12p35, ifnγ-2, arginase 2 and il-10 in the liver of carp sampled in spring. In the liver of carp infected in spring a higher upregulation of the expression of the genes encoding CRPs was observed compared to fish infected during autumn. The opposite trend occurred in the head kidney, where the upregulation of the expression of the genes involved in the immune response was higher in fish infected in autumn than in those infected in spring. During the infection, also season-dependent changes occurred in the estrogen system. In conclusion, we demonstrated that season differentially affects the estrogenic and immune activity of the head kidney and liver. These results reinforce our previous findings that the endocrine and immune systems cooperate in maintaining homeostasis and fighting infection.
Keywords: Common carp; Estrogen system; Head kidney; Immune response; Liver; Seasonality.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
17β-Estradiol affects the innate immune response in common carp.Fish Physiol Biochem. 2020 Oct;46(5):1775-1794. doi: 10.1007/s10695-020-00827-3. Epub 2020 Jun 9. Fish Physiol Biochem. 2020. PMID: 32519008 Free PMC article.
-
Stress differentially affects the systemic and leukocyte estrogen network in common carp.Fish Shellfish Immunol. 2017 Sep;68:190-201. doi: 10.1016/j.fsi.2017.07.011. Epub 2017 Jul 8. Fish Shellfish Immunol. 2017. PMID: 28698119
-
17α-ethinylestradiol and 4-tert-octylphenol concurrently disrupt the immune response of common carp.Fish Shellfish Immunol. 2020 Dec;107(Pt A):238-250. doi: 10.1016/j.fsi.2020.10.005. Epub 2020 Oct 7. Fish Shellfish Immunol. 2020. PMID: 33038508
-
Estrogen-dependent seasonal adaptations in the immune response of fish.Horm Behav. 2017 Feb;88:15-24. doi: 10.1016/j.yhbeh.2016.10.007. Epub 2016 Oct 17. Horm Behav. 2017. PMID: 27760301 Review.
-
Spring viremia of carp (SVC).Dis Aquat Organ. 2002 Dec 10;52(3):261-72. doi: 10.3354/dao052261. Dis Aquat Organ. 2002. PMID: 12553453 Review.
Cited by
-
Seasonal Temperature Differentially Modulates the Immunotranscriptomic Performance in Atlantic Salmon Skin in Response to Natural Caligus rogercresseyi Infestation in Open-Ocean Cages.Animals (Basel). 2025 Aug 12;15(16):2369. doi: 10.3390/ani15162369. Animals (Basel). 2025. PMID: 40867698 Free PMC article.
References
-
- Biswas DK, Singh S, Shi Q et al (2005) Crossroads of estrogen receptor and NF-κB signaling. Sci STKE 2005. 10.1126/stke.2882005pe27 - PubMed
-
- Buchtíková S, Šimková A, Rohlenová K, et al. The seasonal changes in innate immunity of the common carp (Cyprinus carpio) Aquaculture. 2011;318:169–175. doi: 10.1016/j.aquaculture.2011.05.013. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

