Unveiling the dynamics of antimicrobial utilization and resistance in a large hospital network over five years: Insights from health record data analysis
- PMID: 38157341
- PMCID: PMC10756551
- DOI: 10.1371/journal.pdig.0000424
Unveiling the dynamics of antimicrobial utilization and resistance in a large hospital network over five years: Insights from health record data analysis
Abstract
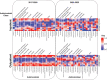
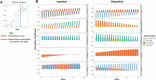
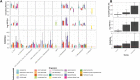
Antimicrobial Resistance (AMR) presents a pressing public health challenge globally which has been compounded by the COVID-19 pandemic. Elucidation of the impact of the pandemic on AMR evolution using population-level data that integrates clinical, laboratory and prescription data remains lacking. Data was extracted from the centralized electronic platform which captures the health records of 60,551 patients with a confirmed infection across the network of public healthcare facilities in Dubai, United Arab Emirates. For all inpatients and outpatients diagnosed with bacterial infection between 01/01/2017 and 31/05/2022, structured and unstructured Electronic Health Record data, microbiological laboratory data including antibiogram, molecular typing and COVID-19 testing information as well as antibiotic prescribing data were extracted curated and linked. Various analytical methods, including time-series analysis, natural language processing (NLP) and unsupervised clustering algorithms, were employed to investigate the trends of antimicrobial usage and resistance over time, assess the impact of prescription practices on resistance rates, and explore the effects of COVID-19 on antimicrobial usage and resistance. Our findings identified a significant impact of COVID-19 on antimicrobial prescription practices, with short-term and long-lasting over-prescription of these drugs. Resistance to antimicrobials increased the odds ratio of all mortality to an average of 2.18 (95% CI: 1.87-2.49) for the most commonly prescribed antimicrobials. Moreover, the effects of antimicrobial prescription practices on resistance were observed within one week of initiation. Significant trends in antimicrobial resistance, exhibiting fluctuations for various drugs and organisms, with an overall increasing trend in resistance levels, particularly post-COVID-19 were identified. This study provides a population-level insight into the evolution of AMR in the context of COVID-19 pandemic. The findings emphasize the impact of COVID-19 on the AMR crisis, which remained evident even two years after the onset of the pandemic. This underscores the necessity for enhanced antimicrobial stewardship to address the evolution of AMR.
Copyright: © 2023 Moradigaravand et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Beović B, Doušak M, Ferreira-Coimbra J, Nadrah K, Rubulotta F, Belliato M, et al. Antibiotic use in patients with COVID-19: a ’snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J Antimicrob Chemother, 2020. 75(11): p. 3386–3390. doi: 10.1093/jac/dkaa326 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
