The next decade of SET: from an oncoprotein to beyond
- PMID: 38157418
- PMCID: PMC11267991
- DOI: 10.1093/jmcb/mjad082
The next decade of SET: from an oncoprotein to beyond
Abstract
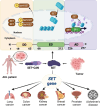
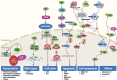
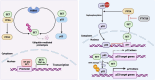
This year marks the fourth decade of research into the protein SET, which was discovered in 1992. SET was initially identified as an oncoprotein but later shown to be a multifaceted protein involved in regulating numerous biological processes under both physiological and pathophysiological conditions. SET dysfunction is closely associated with diseases, such as cancer and Alzheimer's disease. With the increasing understanding of how SET works and how it is regulated in cells, targeting aberrant SET has emerged as a potential strategy for disease intervention. In this review, we present a comprehensive overview of the advancements in SET studies, encompassing its biological functions, regulatory networks, clinical implications, and pharmacological inhibitors. Furthermore, we provide insights into the future prospects of SET research, with a particular emphasis on its promising potential in the realm of immune modulation.
Keywords: SET; biological process; disease; regulatory mechanism; therapy.
© The Author(s) (2023). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures
Similar articles
-
SET Protein in Cancer: A Potential Therapeutic Target.Mini Rev Med Chem. 2021;21(16):2290-2299. doi: 10.2174/1389557521666210114163318. Mini Rev Med Chem. 2021. PMID: 33459234 Review.
-
EVI1 oncoprotein interacts with a large and complex network of proteins and integrates signals through protein phosphorylation.Proc Natl Acad Sci U S A. 2013 Jul 30;110(31):E2885-94. doi: 10.1073/pnas.1309310110. Epub 2013 Jul 15. Proc Natl Acad Sci U S A. 2013. PMID: 23858473 Free PMC article.
-
Emerging role of ZBTB7A as an oncogenic driver and transcriptional repressor.Cancer Lett. 2020 Jul 28;483:22-34. doi: 10.1016/j.canlet.2020.04.015. Epub 2020 Apr 26. Cancer Lett. 2020. PMID: 32348807 Review.
-
Inducing Oncoprotein Degradation to Improve Targeted Cancer Therapy.Neoplasia. 2015 Sep;17(9):697-703. doi: 10.1016/j.neo.2015.08.008. Neoplasia. 2015. PMID: 26476077 Free PMC article. Review.
-
1HN, 13C, and 15N backbone resonance assignments of the SET/TAF-1β/I2PP2A oncoprotein (residues 23-225).Biomol NMR Assign. 2021 Oct;15(2):383-387. doi: 10.1007/s12104-021-10034-7. Epub 2021 Jun 22. Biomol NMR Assign. 2021. PMID: 34156643 Free PMC article.
Cited by
-
Calreticulin and JAK2V617F driver mutations induce distinct mitotic defects in myeloproliferative neoplasms.Sci Rep. 2024 Feb 2;14(1):2810. doi: 10.1038/s41598-024-53240-8. Sci Rep. 2024. PMID: 38308077 Free PMC article.
-
SET facilitates immune escape of microsatellite stability colorectal cancer by inhibiting c-Myc degradation.Cancer Sci. 2025 Jan;116(1):29-43. doi: 10.1111/cas.16368. Epub 2024 Oct 17. Cancer Sci. 2025. PMID: 39420583 Free PMC article.
-
Mir-494-3p enhances aggressive phenotype of non-small cell lung cancer cells by regulating SET/I2PP2A.Sci Rep. 2025 May 2;15(1):15441. doi: 10.1038/s41598-025-99558-9. Sci Rep. 2025. PMID: 40316770 Free PMC article.
-
The oncoprotein SET promotes serine-derived one-carbon metabolism by regulating SHMT2 enzymatic activity.Proc Natl Acad Sci U S A. 2025 May 13;122(19):e2412854122. doi: 10.1073/pnas.2412854122. Epub 2025 May 8. Proc Natl Acad Sci U S A. 2025. PMID: 40339130
References
-
- Adler H.T., Nallaseth F.S., Walter G. et al. (1997). HRX leukemic fusion proteins form a heterocomplex with the leukemia-associated protein SET and protein phosphatase 2A. J. Biol. Chem. 272, 28407–28414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

