Excessive nucleic acid R-loops induce mitochondria-dependent epithelial cell necroptosis and drive spontaneous intestinal inflammation
- PMID: 38157451
- PMCID: PMC10769860
- DOI: 10.1073/pnas.2307395120
Excessive nucleic acid R-loops induce mitochondria-dependent epithelial cell necroptosis and drive spontaneous intestinal inflammation
Abstract
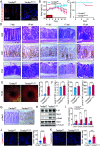
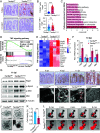
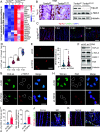
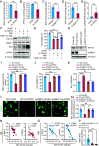
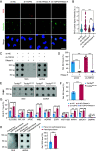
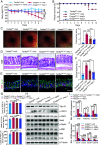
Oxidative stress, which can be activated by a variety of environmental risk factors, has been implicated as an important pathogenic factor for inflammatory bowel disease (IBD). However, how oxidative stress drives IBD onset remains elusive. Here, we found that oxidative stress was strongly activated in inflamed tissues from both ulcerative colitis patients and Crohn's disease patients, and it caused nuclear-to-cytosolic TDP-43 transport and a reduction in the TDP-43 protein level. To investigate the function of TDP-43 in IBD, we inducibly deleted exons 2 to 3 of Tardbp (encoding Tdp-43) in mouse intestinal epithelium, which disrupted its nuclear localization and RNA-processing function. The deletion gave rise to spontaneous intestinal inflammation by inducing epithelial cell necroptosis. Suppression of the necroptotic pathway with deletion of Mlkl or the RIP1 inhibitor Nec-1 rescued colitis phenotypes. Mechanistically, disruption of nuclear TDP-43 caused excessive R-loop accumulation, which triggered DNA damage and genome instability and thereby induced PARP1 hyperactivation, leading to subsequent NAD+ depletion and ATP loss, consequently activating mitochondrion-dependent necroptosis in intestinal epithelial cells. Importantly, restoration of cellular NAD+ levels with NAD+ or NMN supplementation, as well as suppression of ALKBH7, an α-ketoglutarate dioxygenase in mitochondria, rescued TDP-43 deficiency-induced cell death and intestinal inflammation. Furthermore, TDP-43 protein levels were significantly inversely correlated with γ-H2A.X and p-MLKL levels in clinical IBD samples, suggesting the clinical relevance of TDP-43 deficiency-induced mitochondrion-dependent necroptosis. Taken together, these findings identify a unique pathogenic mechanism that links oxidative stress to intestinal inflammation and provide a potent and valid strategy for IBD intervention.
Keywords: R-loop; TDP-43; inflammatory bowel disease; necroptosis.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Arisawa T., et al. , Nrf2 gene promoter polymorphism is associated with ulcerative colitis in a Japanese population. Hepatogastroenterology 55, 394–397 (2008). - PubMed
-
- Karban A., et al. , Paraoxonase (PON)1 192R allele carriage is associated with reduced risk of inflammatory bowel disease. Dig. Dis. Sci. 52, 2707–2715 (2007). - PubMed
-
- Shi X. Z., Lindholm P. F., Sarna S. K., NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology 124, 1369–1380 (2003). - PubMed
MeSH terms
Substances
Grants and funding
- 82025006/MOST | National Natural Science Foundation of China (NSFC)
- 82230017/MOST | National Natural Science Foundation of China (NSFC)
- 82000498/MOST | National Natural Science Foundation of China (NSFC)
- 82270588/MOST | National Natural Science Foundation of China (NSFC)
- 2022YFA1104001/National Basic Research Program of China
- 2022YFC3602102/National Basic Research Program of China
- 2022YFD1300403/National Basic Research Program of China
- 2021YFF1000603/National Basic Research Program of China
- 82300635/MOST | National Natural Science Foundation of China (NSFC)
- 2022M723412/Postdoctoral Science Foundation of China
- 88220019/MOST | National Natural Science Foundation of China (NSFC)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

