Human inherited CCR2 deficiency underlies progressive polycystic lung disease
- PMID: 38157855
- PMCID: PMC10842692
- DOI: 10.1016/j.cell.2023.11.036
Human inherited CCR2 deficiency underlies progressive polycystic lung disease
Erratum in
-
Human inherited CCR2 deficiency underlies progressive polycystic lung disease.Cell. 2024 Jun 20;187(13):3460. doi: 10.1016/j.cell.2024.05.021. Epub 2024 May 21. Cell. 2024. PMID: 38776920 Free PMC article. No abstract available.
Abstract
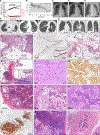
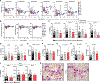
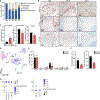
We describe a human lung disease caused by autosomal recessive, complete deficiency of the monocyte chemokine receptor C-C motif chemokine receptor 2 (CCR2). Nine children from five independent kindreds have pulmonary alveolar proteinosis (PAP), progressive polycystic lung disease, and recurrent infections, including bacillus Calmette Guérin (BCG) disease. The CCR2 variants are homozygous in six patients and compound heterozygous in three, and all are loss-of-expression and loss-of-function. They abolish CCR2-agonist chemokine C-C motif ligand 2 (CCL-2)-stimulated Ca2+ signaling in and migration of monocytic cells. All patients have high blood CCL-2 levels, providing a diagnostic test for screening children with unexplained lung or mycobacterial disease. Blood myeloid and lymphoid subsets and interferon (IFN)-γ- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-mediated immunity are unaffected. CCR2-deficient monocytes and alveolar macrophage-like cells have normal gene expression profiles and functions. By contrast, alveolar macrophage counts are about half. Human complete CCR2 deficiency is a genetic etiology of PAP, polycystic lung disease, and recurrent infections caused by impaired CCL2-dependent monocyte migration to the lungs and infected tissues.
Keywords: PAP; chemotaxis; cystic lung disease; macrophage; monocyte; recurrent infection.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.-L.C. serves on the scientific advisory boards of ADMA Biologics Inc., Kymera Therapeutics, and Elixiron Immunotherapeutics. B.C.T. serves as the Scientific Director of the Pulmonary Alveolar Proteinosis Foundation (USA).
Figures




References
-
- Trapnell BC, Whitsett JA, and Nakata K. (2003). Pulmonary alveolar proteinosis. N. Engl. J. Med. 349, 2527–2539. - PubMed
-
- Trapnell BC, Nakata K, Bonella F, Campo I, Griese M, Hamilton J, Wang T, Morgan C, Cottin V, and McCarthy C. (2019). Pulmonary alveolar proteinosis. Nat. Rev. Dis. Primers 5, 16. - PubMed
-
- Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, and Dean M. (2004). ABCA3 gene mutations in newborns with fatal surfactant deficiency. N. Engl. J. Med. 350, 1296–1303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

