CPA toxicity screening of cryoprotective solutions in rat hearts
- PMID: 38158172
- PMCID: PMC11758884
- DOI: 10.1016/j.cryobiol.2023.104842
CPA toxicity screening of cryoprotective solutions in rat hearts
Abstract
In clinical practice, donor hearts are transported on ice prior to transplant and discarded if cold ischemia time exceeds ∼5 h. Methods to extend these preservation times are critically needed, and ideally, this storage time would extend indefinitely, enabling improved donor-to-patient matching, organ utilization, and immune tolerance induction protocols. Previously, we demonstrated successful vitrification and rewarming of whole rat hearts without ice formation by perfusion-loading a cryoprotective agent (CPA) solution prior to vitrification. However, these hearts did not recover any beating even in controls with CPA loading/unloading alone, which points to the chemical toxicity of the cryoprotective solution (VS55 in Euro-Collins carrier solution) as the likely culprit. To address this, we compared the toxicity of another established CPA cocktail (VEG) to VS55 using ex situ rat heart perfusion. The CPA exposure time was 150 min, and the normothermic assessment time was 60 min. Using Celsior as the carrier, we observed partial recovery of function (atria-only beating) for both VS55 and VEG. Upon further analysis, we found that the VEG CPA cocktail resulted in 50 % lower LDH release than VS55 (N = 4, p = 0.017), suggesting VEG has lower toxicity than VS55. Celsior was a better carrier solution than alternatives such as UW, as CPA + Celsior-treated hearts spent less time in cardiac arrest (N = 4, p = 0.029). While we showed substantial improvement in cardiac function after exposure to vitrifiable concentrations of CPA by improving both the CPA and carrier solution formulation, further improvements will be required before we achieve healthy cryopreserved organs for transplant.
Keywords: CPA toxicity; Cryopreservation; Heart; Perfusion; Vitrification.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration competing of interest None.
Figures

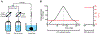



Similar articles
-
Vitrification and Rewarming of Magnetic Nanoparticle-Loaded Rat Hearts.Adv Mater Technol. 2022 Mar;7(3):2100873. doi: 10.1002/admt.202100873. Epub 2021 Oct 1. Adv Mater Technol. 2022. PMID: 35668819 Free PMC article.
-
A comparative study of the most widely used solutions for cardiac graft preservation during hypothermia.J Heart Lung Transplant. 2002 Sep;21(9):1030-9. doi: 10.1016/s1053-2498(02)00414-x. J Heart Lung Transplant. 2002. PMID: 12231375
-
Kidney tissue loading reduces the critical cooling and warming rates of VS55 and VMP cryoprotective solutions.Cryobiology. 2024 Dec;117:104977. doi: 10.1016/j.cryobiol.2024.104977. Epub 2024 Oct 14. Cryobiology. 2024. PMID: 39368531
-
A guide to successful mL to L scale vitrification and rewarming.Cryo Letters. 2022 Nov-Dec;43(6):316-321. Cryo Letters. 2022. PMID: 36629824 Free PMC article. Review.
-
Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures.Cryobiology. 2017 Jun;76:74-91. doi: 10.1016/j.cryobiol.2017.04.004. Epub 2017 Apr 18. Cryobiology. 2017. PMID: 28428046 Review.
References
-
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, Vanwagner LB, Virani SS, Voecks JH, Wang N-Y, Yaffe K, Martin SS, Heart disease and stroke statistics—2022 update: a report from the American heart association, Circulation 145 (2022). - PubMed
-
- Wheeldon D, Sharples L, Wallwork J, English T, Donor heart preservation survey, J. Heart Lung Transplant 11 (1992) 986–993. - PubMed
-
- Goldsmith KA, Demiris N, Gooi JH, Sharples LD, Jenkins DP, Dhital KK, Tsui SS, Life-years gained by reducing donor heart ischemic times, Transplantation 87 (2009) 243–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

