Development of an In Vitro Model to Study Mechanisms of Ultrasound-Targeted Microbubble Cavitation-Mediated Blood-Brain Barrier Opening
- PMID: 38158246
- PMCID: PMC10843834
- DOI: 10.1016/j.ultrasmedbio.2023.12.005
Development of an In Vitro Model to Study Mechanisms of Ultrasound-Targeted Microbubble Cavitation-Mediated Blood-Brain Barrier Opening
Abstract
Objective: Ultrasound-targeted microbubble cavitation (UTMC)-mediated blood-brain barrier (BBB) opening is being explored as a method to increase drug delivery to the brain. This strategy has progressed to clinical trials for various neurological disorders, but the underlying cellular mechanisms are incompletely understood. In the study described here, a contact co-culture transwell model of the BBB was developed that can be used to determine the signaling cascade leading to increased BBB permeability.
Methods: This BBB model consists of bEnd.3 cells and C8-D1A astrocytes seeded on opposite sides of a transwell membrane. Pulsed ultrasound (US) is applied to lipid microbubbles (MBs), and the change in barrier permeability is measured via transendothelial electrical resistance and dextran flux. Live cell calcium imaging (Fluo-4 AM) is performed during UTMC treatment.
Results: This model exhibits important features of the BBB, including endothelial tight junctions, and is more restrictive than the endothelial cell (EC) monolayer alone. When US is applied to MBs in contact with the ECs, BBB permeability increases in this model by two mechanisms: UTMC induces pore formation in the EC membrane (sonoporation), leading to increased transcellular permeability, and UTMC causes formation of reversible inter-endothelial gaps, which increases paracellular permeability. Additionally, this study determines that calcium influx into ECs mediates the increase in BBB permeability after UTMC in this model.
Conclusion: Both transcellular and paracellular permeability can be used to increase drug delivery to the brain. Future studies can use this model to determine how UTMC-induced calcium-mediated signaling increases BBB permeability.
Keywords: Blood–brain barrier; Contrast agent; Microbubble; Sonoporation; Ultrasound.
Copyright © 2023 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures

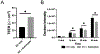
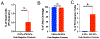



Similar articles
-
The effect of ultrasound pulse length on microbubble cavitation induced antibody accumulation and distribution in a mouse model of breast cancer.Nanotheranostics. 2020 Sep 15;4(4):256-269. doi: 10.7150/ntno.46892. eCollection 2020. Nanotheranostics. 2020. PMID: 33033688 Free PMC article.
-
Reversible Ca2+ signaling and enhanced paracellular transport in endothelial monolayer induced by acoustic bubbles and targeted microbeads.Ultrason Sonochem. 2025 Jan;112:107181. doi: 10.1016/j.ultsonch.2024.107181. Epub 2024 Dec 2. Ultrason Sonochem. 2025. PMID: 39638739 Free PMC article.
-
A multicellular brain spheroid model for studying the mechanisms and bioeffects of ultrasound-enhanced drug penetration beyond the blood‒brain barrier.Sci Rep. 2024 Jan 22;14(1):1909. doi: 10.1038/s41598-023-50203-3. Sci Rep. 2024. PMID: 38253669 Free PMC article.
-
Evaluating the safety profile of focused ultrasound and microbubble-mediated treatments to increase blood-brain barrier permeability.Expert Opin Drug Deliv. 2019 Feb;16(2):129-142. doi: 10.1080/17425247.2019.1567490. Epub 2019 Jan 29. Expert Opin Drug Deliv. 2019. PMID: 30628455 Free PMC article. Review.
-
The road ahead to successful BBB opening and drug-delivery with focused ultrasound.J Control Release. 2024 Aug;372:901-913. doi: 10.1016/j.jconrel.2024.07.006. Epub 2024 Jul 15. J Control Release. 2024. PMID: 38971426 Review.
Cited by
-
Advances in BBB on Chip and Application for Studying Reversible Opening of Blood-Brain Barrier by Sonoporation.Micromachines (Basel). 2022 Dec 30;14(1):112. doi: 10.3390/mi14010112. Micromachines (Basel). 2022. PMID: 36677173 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

