Development of antibody-drug conjugates in cancer: Overview and prospects
- PMID: 38159059
- PMCID: PMC10794012
- DOI: 10.1002/cac2.12517
Development of antibody-drug conjugates in cancer: Overview and prospects
Abstract
In recent years, remarkable breakthroughs have been reported on antibody-drug conjugates (ADCs), with 15 ADCs successfully entering the market over the past decade. This substantial development has positioned ADCs as one of the fastest-growing domains in the realm of anticancer drugs, demonstrating their efficacy in treating a wide array of malignancies. Nonetheless, there is still an unmet clinical need for wider application, better efficacy, and fewer side effects of ADCs. An ADC generally comprises an antibody, a linker and a payload, and the combination has profound effects on drug structure, pharmacokinetic profile and efficacy. Hence, optimization of the key components provides an opportunity to develop ADCs with higher potency and fewer side effects. In this review, we comprehensively reviewed the current development and the prospects of ADC, provided an analysis of marketed ADCs and the ongoing pipelines globally as well as in China, highlighted several ADC platforms and technologies specific to different pharmaceutical enterprises and biotech companies, and also discussed the new related technologies, possibility of next-generation ADCs and the directions of clinical research.
© 2023 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
Rui‐Hua Xu reports speaker fees from Bristol Myers Squibb, Roche, MerckSerono, Hutchison, Hengrui, Junshi, Oilu, CPPC, Henlius, and participates on advisory board for Astellas, MSD, AstraZeneca, Junshi, Hengrui, BeiGene. Innovent, CPPC, and Keymed. All other authors declare no competing interests.
Figures
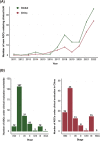
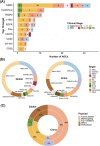

References
-
- Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, et al. Antibody‐drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72(2):165‐182. - PubMed
-
- Chau CH, Steeg PS, Figg WD. Antibody‐drug conjugates for cancer. Lancet. 2019;394(10200):793‐804. - PubMed
-
- Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor‐targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13(17):5043‐5054. - PubMed
-
- Sievers EL, Senter PD. Antibody‐drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15‐29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

