Elimusertib has Antitumor Activity in Preclinical Patient-Derived Pediatric Solid Tumor Models
- PMID: 38159110
- PMCID: PMC10985474
- DOI: 10.1158/1535-7163.MCT-23-0094
Elimusertib has Antitumor Activity in Preclinical Patient-Derived Pediatric Solid Tumor Models
Abstract
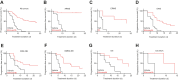
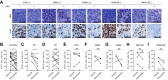
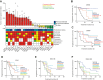
The small-molecule inhibitor of ataxia telangiectasia and Rad3-related protein (ATR), elimusertib, is currently being tested clinically in various cancer entities in adults and children. Its preclinical antitumor activity in pediatric malignancies, however, is largely unknown. We here assessed the preclinical activity of elimusertib in 38 cell lines and 32 patient-derived xenograft (PDX) models derived from common pediatric solid tumor entities. Detailed in vitro and in vivo molecular characterization of the treated models enabled the evaluation of response biomarkers. Pronounced objective response rates were observed for elimusertib monotherapy in PDX, when treated with a regimen currently used in clinical trials. Strikingly, elimusertib showed stronger antitumor effects than some standard-of-care chemotherapies, particularly in alveolar rhabdomysarcoma PDX. Thus, elimusertib has strong preclinical antitumor activity in pediatric solid tumor models, which may translate to clinically meaningful responses in patients.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures



Similar articles
-
Efficacy of ATR Kinase Inhibitor Elimusertib Monotherapy or Combination in Tumors with DNA Damage Response Pathway and Other Genomic Alterations.Mol Cancer Ther. 2025 Sep 2;24(9):1402-1414. doi: 10.1158/1535-7163.MCT-24-0884. Mol Cancer Ther. 2025. PMID: 40300249 Free PMC article.
-
Ovarian and uterine carcinosarcomas are sensitive in vitro and in vivo to elimusertib, a novel ataxia-telangiectasia and Rad3-related (ATR) kinase inhibitor.Gynecol Oncol. 2023 Feb;169:98-105. doi: 10.1016/j.ygyno.2022.12.003. Epub 2022 Dec 14. Gynecol Oncol. 2023. PMID: 36525930 Free PMC article.
-
Broad Spectrum Activity of the Checkpoint Kinase 1 Inhibitor Prexasertib as a Single Agent or Chemopotentiator Across a Range of Preclinical Pediatric Tumor Models.Clin Cancer Res. 2019 Apr 1;25(7):2278-2289. doi: 10.1158/1078-0432.CCR-18-2728. Epub 2018 Dec 18. Clin Cancer Res. 2019. PMID: 30563935 Free PMC article.
-
Systematic Review of Patient-Derived Xenograft Models for Preclinical Studies of Anti-Cancer Drugs in Solid Tumors.Cells. 2019 May 6;8(5):418. doi: 10.3390/cells8050418. Cells. 2019. PMID: 31064068 Free PMC article.
-
Patient-derived xenografts for childhood solid tumors: a valuable tool to test new drugs and personalize treatments.Clin Transl Oncol. 2017 Jan;19(1):44-50. doi: 10.1007/s12094-016-1557-2. Epub 2016 Oct 7. Clin Transl Oncol. 2017. PMID: 27718156 Review.
Cited by
-
Injectable thermosensitive hydrogel co-loading with ATRi and doxorubicin for the treatment of triple-negative breast cancer.RSC Adv. 2025 Jun 16;15(26):20385-20396. doi: 10.1039/d5ra03120f. eCollection 2025 Jun 16. RSC Adv. 2025. PMID: 40530304 Free PMC article.
-
FET fusion oncoproteins disrupt physiologic DNA repair and create a targetable opportunity for ATR inhibitor therapy.bioRxiv [Preprint]. 2025 May 7:2023.04.30.538578. doi: 10.1101/2023.04.30.538578. bioRxiv. 2025. PMID: 37205599 Free PMC article. Preprint.
-
Targeting the DNA damage response in cancer.MedComm (2020). 2024 Oct 31;5(11):e788. doi: 10.1002/mco2.788. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492835 Free PMC article. Review.
-
Therapeutic Targeting of DNA Repair Pathways in Pediatric Extracranial Solid Tumors: Current State and Implications for Immunotherapy.Cancers (Basel). 2024 Apr 25;16(9):1648. doi: 10.3390/cancers16091648. Cancers (Basel). 2024. PMID: 38730598 Free PMC article. Review.
-
Treatment with novel topoisomerase inhibitors in Ewing sarcoma models reveals heterogeneity of tumor response.Front Cell Dev Biol. 2024 Oct 24;12:1462840. doi: 10.3389/fcell.2024.1462840. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39512899 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. - PubMed
-
- Suh E, Stratton KL, Leisenring WM, Nathan PC, Ford JS, Freyer DR, et al. . Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the childhood cancer survivor study. Lancet Oncol 2020;21:421–35. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

