Optimal blood tau species for the detection of Alzheimer's disease neuropathology: an immunoprecipitation mass spectrometry and autopsy study
- PMID: 38159140
- PMCID: PMC10757700
- DOI: 10.1007/s00401-023-02660-3
Optimal blood tau species for the detection of Alzheimer's disease neuropathology: an immunoprecipitation mass spectrometry and autopsy study
Abstract
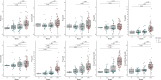
Plasma-to-autopsy studies are essential for validation of blood biomarkers and understanding their relation to Alzheimer's disease (AD) pathology. Few such studies have been done on phosphorylated tau (p-tau) and those that exist have made limited or no comparison of the different p-tau variants. This study is the first to use immunoprecipitation mass spectrometry (IP-MS) to compare the accuracy of eight different plasma tau species in predicting autopsy-confirmed AD. The sample included 123 participants (AD = 69, non-AD = 54) from the Boston University Alzheimer's disease Research Center who had an available ante-mortem plasma sample and donated their brain. Plasma samples proximate to death were analyzed by targeted IP-MS for six different tryptic phosphorylated (p-tau-181, 199, 202, 205, 217, 231), and two non-phosphorylated tau (195-205, 212-221) peptides. NIA-Reagan Institute criteria were used for the neuropathological diagnosis of AD. Binary logistic regressions tested the association between each plasma peptide and autopsy-confirmed AD status. Area under the receiver operating curve (AUC) statistics were generated using predicted probabilities from the logistic regression models. Odds Ratio (OR) was used to study associations between the different plasma tau species and CERAD and Braak classifications. All tau species were increased in AD compared to non-AD, but p-tau217, p-tau205 and p-tau231 showed the highest fold-changes. Plasma p-tau217 (AUC = 89.8), p-tau231 (AUC = 83.4), and p-tau205 (AUC = 81.3) all had excellent accuracy in discriminating AD from non-AD brain donors, even among those with CDR < 1). Furthermore, p-tau217, p-tau205 and p-tau231 showed the highest ORs with both CERAD (ORp-tau217 = 15.29, ORp-tau205 = 5.05 and ORp-tau231 = 3.86) and Braak staging (ORp-tau217 = 14.29, ORp-tau205 = 5.27 and ORp-tau231 = 4.02) but presented increased levels at different amyloid and tau stages determined by neuropathological examination. Our findings support plasma p-tau217 as the most promising p-tau species for detecting AD brain pathology. Plasma p-tau231 and p-tau205 may additionally function as markers for different stages of the disease.
Keywords: Alzheimer’s disease; Autopsy; Biomarkers; Blood; Mass spectrometry; Phosphorylated tau.
© 2023. The Author(s).
Conflict of interest statement
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. PRN participated in Advisory Board for Roche, Novo Nordics and Cerveau (outside submitted work). Andrew E. Budson has served on a consultant or on advisory boards for Sage Pharmaceuticals and Cognito Therapeutics and has received grant monies from Biogen, Bristol Myers Squibb, and Cyclerion. He receives publishing royalties from Elsevier and Oxford University Press. Rhoda Au serves on the scientific advisory board of Signant Health, as consultant to Biogen and has given a lecture in a symposia sponsored by Eisai. Robert A. Stern has served as a consultant to Biogen and Lundbeck. He receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. MLA has received honorarium from the Michael J Fox Foundation for services unrelated to this study. He also receives royalties from Oxford University Press. The other authors declare no competing interests.
Figures


References
-
- Ashton NJ, Janelidze S, Mattsson-Carlgren N, Binette AP, Strandberg O, Brum WS, Karikari TK, González-Ortiz F, Di Molfetta G, Meda FJ, et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat Med. 2022;28:2555–2562. doi: 10.1038/s41591-022-02074-w. - DOI - PMC - PubMed
-
- Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA, Centers NI-AsD The national Alzheimer’s coordinating center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RF1 NS132290/NS/NINDS NIH HHS/United States
- L30 NS093634/NS/NINDS NIH HHS/United States
- P30 AG072978/AG/NIA NIH HHS/United States
- R01 AG068398/AG/NIA NIH HHS/United States
- K23 NS102399/NS/NINDS NIH HHS/United States
- R44 AG063635/AG/NIA NIH HHS/United States
- R01 AG066524/AG/NIA NIH HHS/United States
- RF1 AG075832/AG/NIA NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- IK2 CX002065/CX/CSRD VA/United States
- R01 NS017950/NS/NINDS NIH HHS/United States
- R56 AG062109/AG/NIA NIH HHS/United States
- I01 BX005933/BX/BLRD VA/United States
- U54 NS115266/NS/NINDS NIH HHS/United States
- U19 AG068753/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

