Probing the mechanism by which the retinal G protein transducin activates its biological effector PDE6
- PMID: 38159849
- PMCID: PMC10838916
- DOI: 10.1016/j.jbc.2023.105608
Probing the mechanism by which the retinal G protein transducin activates its biological effector PDE6
Abstract
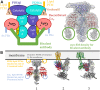
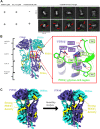
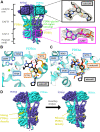
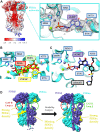
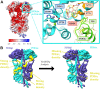
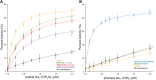
Phototransduction in retinal rods occurs when the G protein-coupled photoreceptor rhodopsin triggers the activation of phosphodiesterase 6 (PDE6) by GTP-bound alpha subunits of the G protein transducin (GαT). Recently, we presented a cryo-EM structure for a complex between two GTP-bound recombinant GαT subunits and native PDE6, that included a bivalent antibody bound to the C-terminal ends of GαT and the inhibitor vardenafil occupying the active sites on the PDEα and PDEβ subunits. We proposed GαT-activated PDE6 by inducing a striking reorientation of the PDEγ subunits away from the catalytic sites. However, questions remained including whether in the absence of the antibody GαT binds to PDE6 in a similar manner as observed when the antibody is present, does GαT activate PDE6 by enabling the substrate cGMP to access the catalytic sites, and how does the lipid membrane enhance PDE6 activation? Here, we demonstrate that 2:1 GαT-PDE6 complexes form with either recombinant or retinal GαT in the absence of the GαT antibody. We show that GαT binding is not necessary for cGMP nor competitive inhibitors to access the active sites; instead, occupancy of the substrate binding sites enables GαT to bind and reposition the PDE6γ subunits to promote catalytic activity. Moreover, we demonstrate by reconstituting GαT-stimulated PDE6 activity in lipid bilayer nanodiscs that the membrane-induced enhancement results from an increase in the apparent binding affinity of GαT for PDE6. These findings provide new insights into how the retinal G protein stimulates rapid catalytic turnover by PDE6 required for dim light vision.
Keywords: G protein; cryoelectron microscopy; phosphodiesterase; phototransduction; signal transduction; structural biology.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest with the contents of this article.
Figures


Similar articles
-
Structure of the Visual Signaling Complex between Transducin and Phosphodiesterase 6.Mol Cell. 2020 Oct 15;80(2):237-245.e4. doi: 10.1016/j.molcel.2020.09.013. Epub 2020 Oct 1. Mol Cell. 2020. PMID: 33007200 Free PMC article.
-
Transducin β-Subunit Can Interact with Multiple G-Protein γ-Subunits to Enable Light Detection by Rod Photoreceptors.eNeuro. 2018 Jun 11;5(3):ENEURO.0144-18.2018. doi: 10.1523/ENEURO.0144-18.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 29911170 Free PMC article.
-
It takes two transducins to activate the cGMP-phosphodiesterase 6 in retinal rods.Open Biol. 2018 Aug;8(8):180075. doi: 10.1098/rsob.180075. Open Biol. 2018. PMID: 30068566 Free PMC article.
-
Photoreceptor phosphodiesterase (PDE6): activation and inactivation mechanisms during visual transduction in rods and cones.Pflugers Arch. 2021 Sep;473(9):1377-1391. doi: 10.1007/s00424-021-02562-x. Epub 2021 Apr 15. Pflugers Arch. 2021. PMID: 33860373 Free PMC article. Review.
-
cGMP Signaling in Photoreceptor Degeneration.Int J Mol Sci. 2023 Jul 7;24(13):11200. doi: 10.3390/ijms241311200. Int J Mol Sci. 2023. PMID: 37446378 Free PMC article. Review.
Cited by
-
Photopic flicker optoretinography captures the light-driven length modulation of photoreceptors during phototransduction.Proc Natl Acad Sci U S A. 2025 Feb 18;122(7):e2421722122. doi: 10.1073/pnas.2421722122. Epub 2025 Feb 13. Proc Natl Acad Sci U S A. 2025. PMID: 39946535 Free PMC article.
-
Structural and functional dynamics of human cone cGMP-phosphodiesterase important for photopic vision.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2419732121. doi: 10.1073/pnas.2419732121. Epub 2024 Dec 31. Proc Natl Acad Sci U S A. 2025. PMID: 39739818 Free PMC article.
References
-
- Burns M.E., Arshavsky V.Y. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

