Palmitoylation of CYSTM (CYSPD) proteins in yeast
- PMID: 38159851
- PMCID: PMC10840359
- DOI: 10.1016/j.jbc.2023.105609
Palmitoylation of CYSTM (CYSPD) proteins in yeast
Abstract
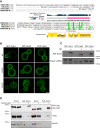
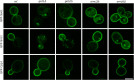
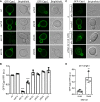
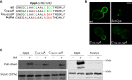
A superfamily of proteins called cysteine transmembrane is widely distributed across eukaryotes. These small proteins are characterized by the presence of a conserved motif at the C-terminal region, rich in cysteines, that has been annotated as a transmembrane domain. Orthologs of these proteins have been involved in resistance to pathogens and metal detoxification. The yeast members of the family are YBR016W, YDL012C, YDR034W-B, and YDR210W. Here, we begin the characterization of these proteins at the molecular level and show that Ybr016w, Ydr034w-b, and Ydr210w are palmitoylated proteins. Protein S-acylation or palmitoylation, is a posttranslational modification that consists of the addition of long-chain fatty acids to cysteine residues. We provide evidence that Ybr016w, Ydr210w, and Ydr034w-b are localized to the plasma membrane and exhibit varying degrees of polarity toward the daughter cell, which is dependent on endocytosis and recycling. We suggest the names CPP1, CPP2, and CPP3 (C terminally palmitoylated protein) for YBR016W, YDR210W, and YDR034W-B, respectively. We show that palmitoylation is responsible for the binding of these proteins to the membrane indicating that the cysteine transmembrane on these proteins is not a transmembrane domain. We propose renaming the C-terminal cysteine-rich domain as cysteine-rich palmitoylated domain. Loss of the palmitoyltransferase Erf2 leads to partial degradation of Ybr016w (Cpp1), whereas in the absence of the palmitoyltransferase Akr1, members of this family are completely degraded. For Cpp1, we show that this degradation occurs via the proteasome in an Rsp5-dependent manner, but is not exclusively due to a lack of Cpp1 palmitoylation.
Keywords: Akr1; CYSPD; CYSTM; Erf2; palmitoylation; yeast.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Andreeva N., Kulakovskaya E., Zvonarev A., Penin A., Eliseeva I., Teterina A., et al. Transcriptome profile of yeast reveals the essential role of PMA2 and uncharacterized gene YBR056W-A (MNC1) in adaptation to toxic manganese concentration. Metallomics. 2017;9:175–182. - PubMed
-
- Beilharz T., Egan B., Silver P.A., Hofmann K., Lithgow T. Bipartite signals mediate subcellular targeting of tail-anchored membrane proteins in Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:8219–8223. - PubMed
-
- Huh W.K., Falvo J.V., Gerke L.C., Carroll A.S., Howson R.W., Weissman J.S., et al. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

