Optimisation of Organ Preservation treatment strategies in patients with rectal cancer with a good clinical response after neoadjuvant (chemo)radiotherapy: Additional contact X-ray brachytherapy versus eXtending the observation period and local excision (OPAXX) - protocol for two multicentre, parallel, single-arm, phase II studies
- PMID: 38159950
- PMCID: PMC10759064
- DOI: 10.1136/bmjopen-2023-076866
Optimisation of Organ Preservation treatment strategies in patients with rectal cancer with a good clinical response after neoadjuvant (chemo)radiotherapy: Additional contact X-ray brachytherapy versus eXtending the observation period and local excision (OPAXX) - protocol for two multicentre, parallel, single-arm, phase II studies
Abstract
Introduction: Standard treatment for patients with intermediate or locally advanced rectal cancer is (chemo)radiotherapy followed by total mesorectal excision (TME) surgery. In recent years, organ preservation aiming at improving quality of life has been explored. Patients with a complete clinical response to (chemo)radiotherapy can be managed safely with a watch-and-wait approach. However, the optimal organ-preserving treatment strategy for patients with a good, but not complete clinical response remains unclear. The aim of the OPAXX study is to determine the rate of organ preservation that can be achieved in patients with rectal cancer with a good clinical response after neoadjuvant (chemo)radiotherapy by additional local treatment options.
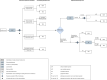
Methods and analysis: The OPAXX study is a Dutch multicentre study that investigates the efficacy of two additional local treatments aiming at organ preservation in patients with a good, but not complete response to neoadjuvant treatment (ie near-complete response or a small residual tumour mass <3 cm). The sample size will be 168 patients in total. Patients will be randomised (1:1) between two parallel single-arm phase II studies: study arm 1 involves additional contact X-ray brachytherapy (an intraluminal radiation boost), while in study arm 2 the observation period is extended followed by a second response evaluation and optional transanal local excision. The primary endpoint of the study is the rate of successful organ preservation at 1 year following randomisation. Secondary endpoints include toxicity, morbidity, oncological and functional outcomes at 1 and 2 years of follow-up. Finally, an observational cohort study for patients who are not eligible for randomisation is conducted.
Ethics and dissemination: The trial protocol has been approved by the medical ethics committee of the Netherlands Cancer Institute (METC20.1276/M20PAX). Informed consent will be obtained from all participants. The trial results will be published in an international peer-reviewed journal.
Trial registration number: NCT05772923.
Keywords: Clinical trials; Colorectal surgery; Gastrointestinal tumours; Radiation oncology.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after Neoadjuvant treatment for Rectal cancer in the International watch & wait database (IWWD): an international Multicentre Registry study. Lancet 2018;391:2537–45. 10.1016/S0140-6736(18)31078-X - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical