A bacteriophage virus-like particle vaccine against oxycodone elicits high-titer and long-lasting antibodies that sequester drug in the blood
- PMID: 38160131
- PMCID: PMC10872394
- DOI: 10.1016/j.vaccine.2023.12.077
A bacteriophage virus-like particle vaccine against oxycodone elicits high-titer and long-lasting antibodies that sequester drug in the blood
Abstract
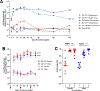
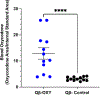
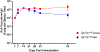
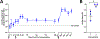
Opioid use disorder (OUD) and opioid overdoses are public health emergencies. In 2021, 80,000 opioid overdose associated deaths were reported in the United States. Despite the availability of treatment strategies, including medications for opioid use disorder (MOUD) and naloxone, opioid overdoses continue to increase at an alarming rate. Opioid vaccines are a novel approach to combat the growing crisis with several candidates recently entering human clinical trials. In this study, we investigated Qβ bacteriophage virus-like particles (VLPs) as a vaccine platform for immunogenic display of oxycodone. A derivative of oxycodone was conjugated to pre-formed Qβ VLPs using a sulfhydryl-amine reactive heterobifunctional crosslinker with high loading of oxycodone. In mice, intramuscular immunization with Qβ-oxycodone elicited high-titer, high-avidity and long-lasting antibody responses. Qβ-oxycodone was also immunogenic after storage at ambient room temperature for over two weeks, demonstrating that the vaccine is highly thermostable. In mice, immunization with Qβ-oxycodone elicited antibodies that sequester oxycodone in the serum, an important mechanism for preventing the adverse effects of opioid activity. Finally, Qβ-oxycodone is immunogenic in nonhuman primates, eliciting serum oxycodone antibodies after intramuscular immunization of rhesus macaques. These data establish Qβ-oxycodone as a promising opioid vaccine candidate.
Keywords: Antibodies; Opioid; Oxycodone; Vaccine; Virus-like particle.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Bryce Chackerian reports financial support was provided by National Heart Lung and Blood Institute. Kathryn M. Frietze reports financial support was provided by National Center for Advancing Translational Sciences. Kathryn M. Frietze reports financial support was provided by Office of Research Infrastructure Program, Office of The Director, National Institutes of Health to the California National Primate Research Center. Isabella G. Romano reports financial support was provided by National Institute of Drug Abuse. Bryce Chackerian reports a relationship with Metaphore Biotechnologies, Inc. that includes: equity or stocks. Kathryn M. Frietze has patent Virus-like particle vaccines for opioid drugs pending to None. Bryce Chackerian has patent Virus-like particle vaccines for opioid drugs. pending to None. Susan B. Core has patent Virus-like particle vaccines for opioid drugs pending to None. Naomi R. Lee has patent Virus-like particle vaccines for opioid drugs pending to None. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- CDC, National Center for Health Statistics, Office of Communication. U.S. Overdose Deaths In 2021 Increased Half as Much as in 2020 – But Are Still Up 15%. CdcGov n.d https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm.
-
- Gardner EA, McGrath SA, Dowling D, Bai D. The Opioid Crisis: Prevalence and Markets of Opioids. Forensic Sci Rev 2022;34:43–70. - PubMed
-
- Commonly Used Terms | CDC’s Response to the Opioid Overdose Epidemic | CDC n.d https://www.cdc.gov/opioids/basics/terms.html (accessed August 25, 2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

