IL-10 dampens antitumor immunity and promotes liver metastasis via PD-L1 induction
- PMID: 38160941
- PMCID: PMC10964083
- DOI: 10.1016/j.jhep.2023.12.015
IL-10 dampens antitumor immunity and promotes liver metastasis via PD-L1 induction
Abstract
Background & aims: The liver is one of the organs most commonly affected by metastasis. The presence of liver metastases has been reported to be responsible for an immunosuppressive microenvironment and diminished immunotherapy efficacy. Herein, we aimed to investigate the role of IL-10 in liver metastasis and to determine how its modulation could affect the efficacy of immunotherapy in vivo.
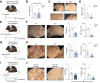
Methods: To induce spontaneous or forced liver metastasis in mice, murine cancer cells (MC38) or colon tumor organoids were injected into the cecum or the spleen, respectively. Mice with complete and cell type-specific deletion of IL-10 and IL-10 receptor alpha were used to identify the source and the target of IL-10 during metastasis formation. Programmed death ligand 1 (PD-L1)-deficient mice were used to test the role of this checkpoint. Flow cytometry was applied to characterize the regulation of PD-L1 by IL-10.
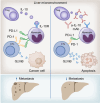
Results: We found that Il10-deficient mice and mice treated with IL-10 receptor alpha antibodies were protected against liver metastasis formation. Furthermore, by using IL-10 reporter mice, we demonstrated that Foxp3+ regulatory T cells (Tregs) were the major cellular source of IL-10 in liver metastatic sites. Accordingly, deletion of IL-10 in Tregs, but not in myeloid cells, led to reduced liver metastasis. Mechanistically, IL-10 acted on Tregs in an autocrine manner, thereby further amplifying IL-10 production. Furthermore, IL-10 acted on myeloid cells, i.e. monocytes, and induced the upregulation of the immune checkpoint protein PD-L1. Finally, the PD-L1/PD-1 axis attenuated CD8-dependent cytotoxicity against metastatic lesions.
Conclusions: Treg-derived IL-10 upregulates PD-L1 expression in monocytes, which in turn reduces CD8+ T-cell infiltration and related antitumor immunity in the context of colorectal cancer-derived liver metastases. These findings provide the basis for future monitoring and targeting of IL-10 in colorectal cancer-derived liver metastases.
Impact and implications: Liver metastasis diminishes the effectiveness of immunotherapy and increases the mortality rate in patients with colorectal cancer. We investigated the role of IL-10 in liver metastasis formation and assessed its impact on the effectiveness of immunotherapy. Our data show that IL-10 is a pro-metastatic factor involved in liver metastasis formation and that it acts as a regulator of PD-L1. This provides the basis for future monitoring and targeting of IL-10 in colorectal cancer-derived liver metastasis.
Keywords: IL-10; PD-L1; immune cells; immunotherapy; liver metastasis.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clinicians. 2018;68:394–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

