Multifunctional nanoparticle-mediated combining therapy for human diseases
- PMID: 38161204
- PMCID: PMC10758001
- DOI: 10.1038/s41392-023-01668-1
Multifunctional nanoparticle-mediated combining therapy for human diseases
Abstract
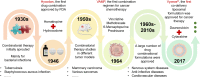
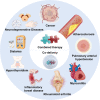
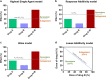
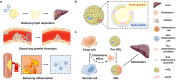
Combining existing drug therapy is essential in developing new therapeutic agents in disease prevention and treatment. In preclinical investigations, combined effect of certain known drugs has been well established in treating extensive human diseases. Attributed to synergistic effects by targeting various disease pathways and advantages, such as reduced administration dose, decreased toxicity, and alleviated drug resistance, combinatorial treatment is now being pursued by delivering therapeutic agents to combat major clinical illnesses, such as cancer, atherosclerosis, pulmonary hypertension, myocarditis, rheumatoid arthritis, inflammatory bowel disease, metabolic disorders and neurodegenerative diseases. Combinatorial therapy involves combining or co-delivering two or more drugs for treating a specific disease. Nanoparticle (NP)-mediated drug delivery systems, i.e., liposomal NPs, polymeric NPs and nanocrystals, are of great interest in combinatorial therapy for a wide range of disorders due to targeted drug delivery, extended drug release, and higher drug stability to avoid rapid clearance at infected areas. This review summarizes various targets of diseases, preclinical or clinically approved drug combinations and the development of multifunctional NPs for combining therapy and emphasizes combinatorial therapeutic strategies based on drug delivery for treating severe clinical diseases. Ultimately, we discuss the challenging of developing NP-codelivery and translation and provide potential approaches to address the limitations. This review offers a comprehensive overview for recent cutting-edge and challenging in developing NP-mediated combination therapy for human diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Co-delivery of Docetaxel and Disulfonate Tetraphenyl Chlorin in One Nanoparticle Produces Strong Synergism between Chemo- and Photodynamic Therapy in Drug-Sensitive and -Resistant Cancer Cells.Mol Pharm. 2018 Oct 1;15(10):4599-4611. doi: 10.1021/acs.molpharmaceut.8b00597. Epub 2018 Sep 7. Mol Pharm. 2018. PMID: 30148955
-
Nanoparticle-Mediated Drug Delivery Systems For The Treatment Of IBD: Current Perspectives.Int J Nanomedicine. 2019 Nov 13;14:8875-8889. doi: 10.2147/IJN.S210315. eCollection 2019. Int J Nanomedicine. 2019. PMID: 32009785 Free PMC article. Review.
-
Biodegradable, polymeric nanoparticle delivery systems for cancer therapy.Nanomedicine (Lond). 2007 Oct;2(5):669-80. doi: 10.2217/17435889.2.5.669. Nanomedicine (Lond). 2007. PMID: 17976029 Review.
-
Internal cross-linked polymeric nanoparticles with dual sensitivity for combination therapy of muscle-invasive bladder cancer.J Nanobiotechnology. 2020 Sep 4;18(1):124. doi: 10.1186/s12951-020-00686-3. J Nanobiotechnology. 2020. PMID: 32887622 Free PMC article.
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
Cited by
-
Integrated analysis of cell cycle and p53 signaling pathways related genes in breast, colorectal, lung, and pancreatic cancers: implications for prognosis and drug sensitivity for therapeutic potential.Discov Oncol. 2024 Dec 23;15(1):832. doi: 10.1007/s12672-024-01712-8. Discov Oncol. 2024. PMID: 39715832 Free PMC article.
-
Editorial: Enhancing innate immunity in combination therapy for solid tumors.Front Immunol. 2025 Jan 3;15:1539027. doi: 10.3389/fimmu.2024.1539027. eCollection 2024. Front Immunol. 2025. PMID: 39830515 Free PMC article. No abstract available.
-
Circular RNAs in cancer.MedComm (2020). 2025 Feb 2;6(2):e70079. doi: 10.1002/mco2.70079. eCollection 2025 Feb. MedComm (2020). 2025. PMID: 39901896 Free PMC article. Review.
-
Stimuli-Responsive Nodal Dual-Drug Polymer Nanoparticles for Cancer Therapy.Int J Nanomedicine. 2025 Apr 23;20:5181-5192. doi: 10.2147/IJN.S517291. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40292406 Free PMC article.
-
Enhancing hepatocellular carcinoma treatment: synergistic cytotoxicity and mechanistic insights of Ramucirumab and 5-Azacytidine combination therapy.Saudi Pharm J. 2025 Apr 29;33(1-2):6. doi: 10.1007/s44446-025-00001-x. Saudi Pharm J. 2025. PMID: 40397219 Free PMC article.
References
-
- Shrestha B, Tang L, Romero G. Nanoparticles‐mediated combination therapies for cancer treatment. Adv. Ther. 2019;2:1900076. doi: 10.1002/adtp.201900076. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

