Photo-crosslinked bioactive BG/BMSCs@GelMA hydrogels for bone-defect repairs
- PMID: 38161508
- PMCID: PMC10755535
- DOI: 10.1016/j.mtbio.2023.100882
Photo-crosslinked bioactive BG/BMSCs@GelMA hydrogels for bone-defect repairs
Abstract
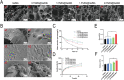
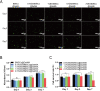
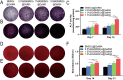
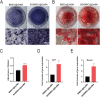
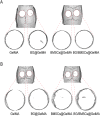
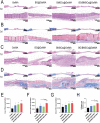
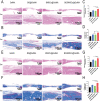
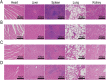
The clinical treatments of bone defects remain a challenge. Hydrogels containing bone marrow mesenchymal stem cells (BMSCs) are extensively used to bone regeneration because of excellent biocompatibility and hydrophilicity. However, the insufficient osteo-induction capacity of the BMSC-loaded hydrogels limits their clinical applications. In this study, bio-active glass (BG) and BMSCs were combined with gelatin methacryloyl (GelMA) to fabricate composite hydrogels via photo-crosslinking, and the regulation of bone regeneration was investigated. In vitro experiments showed that the BG/BMSCs@GelMA hydrogel had excellent cytocompatibility and promoted osteogenic differentiation in BMSCs. Furthermore, the BG/BMSCs@GelMA hydrogel was injected into critical-sized calvarial defects, and the results further confirmed its excellent angiogenetic and bone regeneration capacity. In addition, BG/BMSCs@GelMA promoted the polarization of macrophages towards the M2 phenotype. In summary, this novel composite hydrogel demonstrated remarkable potential for application in bone regeneration due to its immunomodulatory, excellent angiogenetic as well as osteo-induction capacity.
Keywords: BMSCs; Bio-active glass; Bone regeneration; GelMA.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Cerium Oxide Nanoparticles-Reinforced GelMA Hydrogel Loading Bone Marrow Stem Cells with Osteogenic and Inflammatory Regulatory Capacity for Bone Defect Repair.ACS Appl Mater Interfaces. 2024 Dec 11;16(49):67373-67384. doi: 10.1021/acsami.4c15718. Epub 2024 Nov 25. ACS Appl Mater Interfaces. 2024. PMID: 39585753
-
Injectable Photo-Crosslinked Bioactive BMSCs-BMP2-GelMA Scaffolds for Bone Defect Repair.Front Bioeng Biotechnol. 2022 Mar 24;10:875363. doi: 10.3389/fbioe.2022.875363. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35402421 Free PMC article.
-
Synthesis of Silanized Bioactive Glass/Gelatin Methacrylate (GelMA/Si-BG) composite hydrogel for Bone Tissue Engineering Application.J Mech Behav Biomed Mater. 2023 Nov;147:106159. doi: 10.1016/j.jmbbm.2023.106159. Epub 2023 Sep 30. J Mech Behav Biomed Mater. 2023. PMID: 37797555
-
GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances.Biomater Res. 2023 Sep 15;27(1):86. doi: 10.1186/s40824-023-00422-6. Biomater Res. 2023. PMID: 37715230 Free PMC article. Review.
-
Advanced Hybrid Strategies of GelMA Composite Hydrogels in Bone Defect Repair.Polymers (Basel). 2024 Oct 29;16(21):3039. doi: 10.3390/polym16213039. Polymers (Basel). 2024. PMID: 39518248 Free PMC article. Review.
Cited by
-
Metal-phenolic network biointerface-mediated cell regulation for bone tissue regeneration.Mater Today Bio. 2024 Dec 12;30:101400. doi: 10.1016/j.mtbio.2024.101400. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39759849 Free PMC article. Review.
-
Curcumin-encapsulated exosomes in bisphosphonate-modified hydrogel microspheres promote bone repair through macrophage polarization and DNA damage mitigation.Mater Today Bio. 2025 May 15;32:101874. doi: 10.1016/j.mtbio.2025.101874. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40487156 Free PMC article.
-
Advances in hybrid hydrogel design for biomedical applications: innovations in drug delivery and tissue engineering for gynecological cancers.Cell Biol Toxicol. 2025 Jul 12;41(1):115. doi: 10.1007/s10565-025-10064-0. Cell Biol Toxicol. 2025. PMID: 40646356 Free PMC article. Review.
-
Gelatin/Cerium-Doped Bioactive Glass Composites for Enhancing Cellular Functions of Human Mesenchymal Stem Cells (hBMSCs).Gels. 2025 Jun 1;11(6):425. doi: 10.3390/gels11060425. Gels. 2025. PMID: 40558726 Free PMC article.
-
Multifunctional Hydrogel with Synergistic Reactive Oxygen Species Scavenging and Macrophage Polarization-Induced Osteo-immunomodulation for Enhanced Bone Regeneration.ACS Appl Mater Interfaces. 2025 Jul 9;17(27):38985-39001. doi: 10.1021/acsami.5c08737. Epub 2025 Jun 24. ACS Appl Mater Interfaces. 2025. PMID: 40552867 Free PMC article.
References
-
- Sun Y., Liu X., Zhu Y., Han Y., Shen J., Bao B., Gao T., Lin J., Huang T., Xu J., Chai Y., Zheng X. Tunable and controlled release of cobalt ions from metal-organic framework hydrogel nanocomposites enhances bone regeneration. ACS Appl. Mater. Inter. 2021;13:16. - PubMed
LinkOut - more resources
Full Text Sources

