Melanin/melanin-like nanoparticles: As a naturally active platform for imaging-guided disease therapy
- PMID: 38161509
- PMCID: PMC10755544
- DOI: 10.1016/j.mtbio.2023.100894
Melanin/melanin-like nanoparticles: As a naturally active platform for imaging-guided disease therapy
Abstract
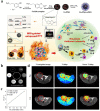
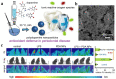
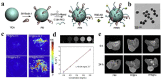
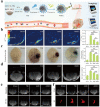
The development of biocompatible and efficient nanoplatforms that combine diagnostic and therapeutic functions is of great importance for precise disease treatment. Melanin, an endogenous biopolymer present in living organisms, has attracted increasing attention as a versatile bioinspired functional platform owing to its unique physicochemical properties (e.g., high biocompatibility, strong chelation of metal ions, broadband light absorption, high drug binding properties) and inherent antioxidant, photoprotective, anti-inflammatory, and anti-tumor effects. In this review, the fundamental physicochemical properties and preparation methods of natural melanin and melanin-like nanoparticles were outlined. A systematical description of the recent progress of melanin and melanin-like nanoparticles in single, dual-, and tri-multimodal imaging-guided the visual administration and treatment of osteoarthritis, acute liver injury, acute kidney injury, acute lung injury, brain injury, periodontitis, iron overload, etc. Was then given. Finally, it concluded with a reasoned discussion of current challenges toward clinical translation and future striving directions. Therefore, this comprehensive review provides insight into the current status of melanin and melanin-like nanoparticles research and is expected to optimize the design of novel melanin-based therapeutic platforms and further clinical translation.
Keywords: Imaging-guided; Melanin-like nanoparticles; Therapeutic platform; melanin nanoparticles.
© 2023 The Authors.
Conflict of interest statement
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Figures





Similar articles
-
Melanin-Like Nanomaterials for Advanced Biomedical Applications: A Versatile Platform with Extraordinary Promise.Adv Sci (Weinh). 2020 Feb 7;7(7):1903129. doi: 10.1002/advs.201903129. eCollection 2020 Apr. Adv Sci (Weinh). 2020. PMID: 32274309 Free PMC article. Review.
-
Melanin and Melanin-Functionalized Nanoparticles as Promising Tools in Cancer Research-A Review.Cancers (Basel). 2022 Apr 6;14(7):1838. doi: 10.3390/cancers14071838. Cancers (Basel). 2022. PMID: 35406610 Free PMC article. Review.
-
Melanin-based nanomaterials: The promising nanoplatforms for cancer diagnosis and therapy.Nanomedicine. 2020 Aug;28:102211. doi: 10.1016/j.nano.2020.102211. Epub 2020 Apr 19. Nanomedicine. 2020. PMID: 32320736 Review.
-
An auto-photoacoustic melanin-based drug delivery nano-platform for self-monitoring of acute kidney injury therapy via a triple-collaborative strategy.Acta Biomater. 2022 Jul 15;147:327-341. doi: 10.1016/j.actbio.2022.05.034. Epub 2022 May 25. Acta Biomater. 2022. PMID: 35643195
-
Melanin nanoparticles as a promising tool for biomedical applications - a review.Acta Biomater. 2020 Mar 15;105:26-43. doi: 10.1016/j.actbio.2020.01.044. Epub 2020 Feb 1. Acta Biomater. 2020. PMID: 32014585 Review.
Cited by
-
Implementing differentially pigmented skin models for predicting drug response variability across human ancestries.Hum Genomics. 2024 Oct 9;18(1):113. doi: 10.1186/s40246-024-00677-7. Hum Genomics. 2024. PMID: 39385300 Free PMC article. Review.
-
Capsaicin-Loaded Melanin Nanoparticles for Long-Lasting Nociceptive-Selective Nerve Blockade.Langmuir. 2024 Nov 26;40(47):24751-24760. doi: 10.1021/acs.langmuir.4c01725. Epub 2024 Nov 14. Langmuir. 2024. PMID: 39541506 Free PMC article.
-
Strategies for specific multimodal imaging of cancer-associated fibroblasts and applications in theranostics of cancer.Mater Today Bio. 2024 Dec 24;30:101420. doi: 10.1016/j.mtbio.2024.101420. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39839493 Free PMC article. Review.
-
Innovative nanocarriers: Synthetic and biomimetic strategies for enhanced drug delivery.Mater Today Bio. 2025 Aug 8;34:102180. doi: 10.1016/j.mtbio.2025.102180. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40838213 Free PMC article. Review.
-
Multienzyme active melanin nanodots for antioxidant-immunomodulatory therapy of hyperoxia lung injury.Mater Today Bio. 2025 Feb 25;31:101609. doi: 10.1016/j.mtbio.2025.101609. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40104637 Free PMC article.
References
-
- Chen Q., et al. Recent advances in different modal imaging-guided photothermal therapy. Biomaterials. 2016;106:144–166. - PubMed
-
- Chetrit M., et al. Imaging-guided therapies for pericardial diseases. JACC Cardiovasc Imaging. 2020;13(6):1422–1437. - PubMed
-
- Xie B., et al. Supramolecular biomaterials for bio-imaging and imaging-guided therapy. Eur. J. Nucl. Med. Mol. Imag. 2022;49(4):1200–1210. - PubMed
-
- Lim E.K., et al. Nanomaterials for theranostics: recent advances and future challenges. Chem Rev. 2015;115(1):327–394. - PubMed
-
- Chen G., et al. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116(5):2826–2885. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

