An infection-microenvironment-targeted and responsive peptide-drug nanosystem for sepsis emergency by suppressing infection and inflammation
- PMID: 38161786
- PMCID: PMC10755722
- DOI: 10.1016/j.ajps.2023.100869
An infection-microenvironment-targeted and responsive peptide-drug nanosystem for sepsis emergency by suppressing infection and inflammation
Abstract
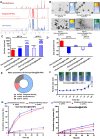
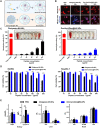
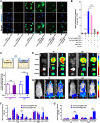
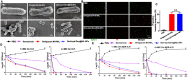
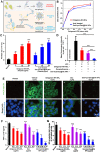
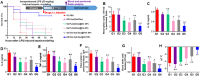
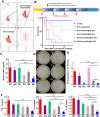
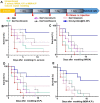
Sepsis is a life-threatening emergency that causes millions of deaths every year due to severe infection and inflammation. Nevertheless, current therapeutic regimens are inadequate to promptly address the vast diversity of potential pathogens. Omiganan, an antimicrobial peptide, has shown promise for neutralizing endotoxins and eliminating diverse pathogens. However, its clinical application is hindered by safety and stability concerns. Herein, we present a nanoscale drug delivery system (Omi-hyd-Dex@HA NPs) that selectively targets infectious microenvironments (IMEs) and responds to specific stimuli for efficient intervention in sepsis. The system consists of omiganan-dexamethasone conjugates linked by hydrazone bonds which self-assemble into nanoparticles coated with a hyaluronic acid (HA). The HA coating not only facilitates IMEs-targeting through interaction with intercellular-adhesion-molecule-1 on inflamed endotheliocytes, but also improves the biosafety of the nanosystem and enhances drug accumulation in primary infection sites triggered by hyaluronidase. The nanoparticles release dual drugs in IMEs through pH-sensitive cleavage of hydrazone bonds to eradicate pathogens and suppress inflammation. In multiple tissue infection and sepsis animal models, Omi-hyd-Dex@HA NPs exhibited rapid source control and comprehensive inflammation reduction, thereby preventing subsequent fatal complications and significantly improving survival outcomes. The bio-responsive and self-delivering nanosystem offers a promising strategy for systemic sepsis treatment in emergencies.
Keywords: Infectious microenvironments; Nanoscale drug delivery systems; Omiganan; Pathogens; Sepsis.
© 2023 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Figures


Similar articles
-
Bioresponsive Nanoparticles Targeted to Infectious Microenvironments for Sepsis Management.Adv Mater. 2018 Oct;30(43):e1803618. doi: 10.1002/adma.201803618. Epub 2018 Sep 11. Adv Mater. 2018. PMID: 30203430 Free PMC article.
-
Targeted co-delivery of a photosensitizer and an antisense oligonucleotide based on an activatable hyaluronic acid nanosystem with endogenous oxygen generation for enhanced photodynamic therapy of hypoxic tumors.Acta Biomater. 2022 Nov;153:419-430. doi: 10.1016/j.actbio.2022.09.025. Epub 2022 Sep 14. Acta Biomater. 2022. PMID: 36115655
-
Tumor-targeting pH/redox dual-responsive nanosystem epigenetically reverses cancer drug resistance by co-delivering doxorubicin and GCN5 siRNA.Acta Biomater. 2021 Nov;135:556-566. doi: 10.1016/j.actbio.2021.09.002. Epub 2021 Sep 5. Acta Biomater. 2021. PMID: 34496281
-
Inflammation-responsive drug delivery nanosystems for treatment of bacterial-induced sepsis.Int J Pharm. 2023 Sep 25;644:123346. doi: 10.1016/j.ijpharm.2023.123346. Epub 2023 Aug 25. Int J Pharm. 2023. PMID: 37633537 Review.
-
Stimuli-responsive and biomimetic delivery systems for sepsis and related complications.J Control Release. 2022 Dec;352:1048-1070. doi: 10.1016/j.jconrel.2022.11.013. Epub 2022 Nov 17. J Control Release. 2022. PMID: 36372385 Review.
Cited by
-
Progress in macrophage immune regulation of atherosclerosis.Am J Transl Res. 2025 May 15;17(5):3261-3275. doi: 10.62347/GMTC2479. eCollection 2025. Am J Transl Res. 2025. PMID: 40535630 Free PMC article. Review.
-
Regulatory Role and Mechanism of lncRNA RNF217-AS1 in the Proliferation and Migration of Esophageal Cancer Cells.Cancer Manag Res. 2025 Jul 7;17:1329-1337. doi: 10.2147/CMAR.S515036. eCollection 2025. Cancer Manag Res. 2025. PMID: 40656134 Free PMC article.
-
Natural hydrogen gas and engineered microalgae prevent acute lung injury in sepsis.Mater Today Bio. 2024 Sep 14;28:101247. doi: 10.1016/j.mtbio.2024.101247. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39328786 Free PMC article.
-
NIR triggered polydopamine coated cerium dioxide nanozyme for ameliorating acute lung injury via enhanced ROS scavenging.J Nanobiotechnology. 2024 Jun 8;22(1):321. doi: 10.1186/s12951-024-02570-w. J Nanobiotechnology. 2024. PMID: 38849841 Free PMC article.
-
Forging a New Frontier: Antimicrobial Peptides and Nanotechnology Converging to Conquer Gastrointestinal Pathogens.Small. 2025 Jul;21(26):e2501431. doi: 10.1002/smll.202501431. Epub 2025 May 19. Small. 2025. PMID: 40384187 Free PMC article. Review.
References
-
- Cecconi M., Evans L., Levy M., Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. - PubMed
LinkOut - more resources
Full Text Sources

