Optimization of pharmacological interventions in the guinea pig animal model-a new approach to calculate the perilymph volume of the scala tympani
- PMID: 38161797
- PMCID: PMC10754993
- DOI: 10.3389/fnins.2023.1297046
Optimization of pharmacological interventions in the guinea pig animal model-a new approach to calculate the perilymph volume of the scala tympani
Abstract
Objective: The guinea pig serves as a well-established animal model for inner ear research, offering valuable insights into the anatomy, physiology, and therapeutic interventions of the auditory system. However, the heterogeneity of results observed in both in-vivo experiments and clinical studies poses challenges in understanding and optimizing pharmacotherapy outcomes. This heterogeneity may be due to individual differences in the size of the guinea pig cochlea and thus in the volume of the scala tympani (ST), which can lead to different drug concentrations in the ST, a fact that has been largely overlooked thus far. To address this issue, we aimed to develop an approach for calculating the individual volume of perilymph within the ST before and after cochlear implant insertion.
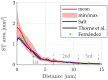
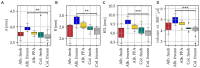
Method: In this study, high-resolution μCT images of a total of n = 42 guinea pig temporal bones were used to determine the volume of the ST. We compared fresh, frozen, and fixed tissues from both colored and albino strains to evaluate the potential influence of tissue condition and strain on the results.
Results: Our findings demonstrate a variability in mean ST volume with a relative standard deviation (RSD) of 14.7%, comparable to studies conducted with humans (range RSD: 5 to 20%). This indicates that the guinea pig cochlea exhibits similar variability to that of the human cochlea. Consequently, it is crucial to consider this variability when designing and conducting studies utilizing the guinea pig as an animal model. Furthermore, we successfully developed a tool capable of estimating ST volume without the need for manual segmentation, employing two geometric parameters, basal diameter (A) and width (B) of the cochlea, corresponding to the cochlear footprint. The tool is available for free download and use on our website.
Conclusion: This novel approach provides researchers with a valuable tool to calculate individual ST volume in guinea pigs, enabling more precise dosing strategies and optimization of drug concentrations for pharmacotherapy studies. Moreover, our study underscores the importance of acknowledging and accounting for inter-individual variability in animal models to enhance the translational relevance and applicability of research outcomes in the field of inner ear investigations.
Keywords: cochlear implantation; cochlear modeling; drug delivery; electrode coating; individualized implantation; pharmacokinetics.
Copyright © 2023 Grzybowski, Malfeld, Lenarz, Scheper and Schurzig.
Conflict of interest statement
The CI electrodes employed for the present investigations were supplied by MED-EL. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Perilymph sampling from the cochlear apex: a reliable method to obtain higher purity perilymph samples from scala tympani.J Neurosci Methods. 2006 May 15;153(1):121-9. doi: 10.1016/j.jneumeth.2005.10.008. Epub 2005 Nov 28. J Neurosci Methods. 2006. PMID: 16310856 Free PMC article.
-
Cochlear Size Assessment Predicts Scala Tympani Volume and Electrode Insertion Force- Implications in Robotic Assisted Cochlear Implant Surgery.Front Surg. 2021 Sep 30;8:723897. doi: 10.3389/fsurg.2021.723897. eCollection 2021. Front Surg. 2021. PMID: 34660676 Free PMC article.
-
Cochlear pharmacokinetics of cisplatin: an in vivo study in the guinea pig.Laryngoscope. 2013 Dec;123(12):3172-7. doi: 10.1002/lary.24235. Epub 2013 Jul 29. Laryngoscope. 2013. PMID: 23754209
-
Potassium circulation in the perilymph of guinea pig cochlea.Eur Arch Otorhinolaryngol. 1994;251 Suppl 1:S48-52. doi: 10.1007/BF02565219. Eur Arch Otorhinolaryngol. 1994. PMID: 11894775
-
Quantification of solute entry into cochlear perilymph through the round window membrane.Hear Res. 2001 Apr;154(1-2):88-97. doi: 10.1016/s0378-5955(01)00223-4. Hear Res. 2001. PMID: 11423219
References
-
- Ahmadi N., Gausterer J. C., Honeder C., Moetz M., Schöpper H., Zhu C., et al. . (2019). Long-term effects and potential limits of intratympanic dexamethasone-loaded hydrogels combined with dexamethasone-eluting cochlear electrodes in a low-insertion trauma Guinea pig model. Hear. Res. 384:107825. doi: 10.1016/j.heares.2019.107825 - DOI - PubMed
LinkOut - more resources
Full Text Sources

