Receptor-interacting protein 1 and 3 kinase activity are required for high-fat diet induced liver injury in mice
- PMID: 38161978
- PMCID: PMC10757356
- DOI: 10.3389/fendo.2023.1267996
Receptor-interacting protein 1 and 3 kinase activity are required for high-fat diet induced liver injury in mice
Abstract
Background: The RIP1-RIP3-MLKL-mediated cell death pathway is associated with progression of non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Previous work identified a critical role for MLKL, the key effector regulating necroptosis, but not RIP3, in mediating high fat diet-induced liver injury in mice. RIP1 and RIP3 have active N-terminus kinase domains essential for activation of MLKL and subsequent necroptosis. However, little is known regarding domain-specific roles of RIP1/RIP3 kinase in liver diseases. Here, we hypothesized that RIP1/RIP3 kinase activity are required for the development of high fat diet-induced liver injury.
Methods: Rip1K45A/K45A and Rip3K51A/K51A kinase-dead mice on a C57BL/6J background and their littermate controls (WT) were allowed free access to a diet high in fat, fructose and cholesterol (FFC diet) or chow diet.
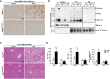
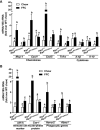
Results: Both Rip1K45A/K45A and Rip3K51A/K51A mice were protected against FFC diet-induced steatosis, hepatocyte injury and expression of hepatic inflammatory cytokines and chemokines. FFC diet increased phosphorylation and oligomerization of MLKL and hepatocyte death in livers of WT, but not in Rip3K51A/K51A, mice. Consistent with in vivo data, RIP3 kinase deficiency in primary hepatocytes prevented palmitic acid-induced translocation of MLKL to the cell surface and cytotoxicity. Additionally, loss of Rip1 or Rip3 kinase suppressed FFC diet-mediated formation of crown-like structures (indicators of dead adipocytes) and expression of mRNA for inflammatory response genes in epididymal adipose tissue. Moreover, FFC diet increased expression of multiple adipokines, including leptin and plasminogen activator inhibitor 1, in WT mice, which was abrogated by Rip3 kinase deficiency.
Discussion: The current data indicate that both RIP1 and RIP3 kinase activity contribute to FFC diet-induced liver injury. This effect of RIP1 and RIP3 kinase deficiency on injury is consistent with the protection of Mlkl-/- mice from high fat diet-induced liver injury, but not the reported lack of protection in Rip3-/- mice. Taken together with previous reports, our data suggest that other domains of RIP3 likely counteract the effect of RIP3 kinase in response to high fat diets.
Keywords: FFC diet; NAFLD; RIP1 kinase; RIP3 kinase; cell death; obesity.
Copyright © 2023 Wu, Arya, Huang, McMullen and Nagy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

