Cerebrospinal fluid exploratory proteomics and ketamine metabolite pharmacokinetics in human volunteers after ketamine infusion
- PMID: 38162029
- PMCID: PMC10755719
- DOI: 10.1016/j.isci.2023.108527
Cerebrospinal fluid exploratory proteomics and ketamine metabolite pharmacokinetics in human volunteers after ketamine infusion
Abstract
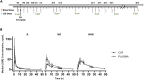
Ketamine is a treatment for both refractory depression and chronic pain syndromes. In order to explore ketamine's potential mechanism of action and whether ketamine or its metabolites cross the blood brain barrier, we examined the pharmacokinetics of ketamine and its metabolites-norketamine (NK), dehydronorketamine (DHNK), and hydroxynorketamines (HNKs)-in cerebrospinal fluid (CSF) and plasma, as well as in an exploratory proteomic analysis in the CSF of nine healthy volunteers who received ketamine intravenously (0.5 mg/kg IV). We found that ketamine, NK, and (2R,6R;2S,6S)-HNK readily crossed the blood brain barrier. Additionally, 354 proteins were altered in the CSF in at least two consecutive timepoints (p < 0.01). Proteins in the classes of tyrosine kinases, cellular adhesion molecules, and growth factors, including insulin, were most affected, suggesting an interplay of altered neurotransmission, neuroplasticity, neurogenesis, synaptogenesis, and neural network functions following ketamine administration.
Keywords: Health sciences; Medicine; Pharmacology; Psychiatry.
© 2023.
Conflict of interest statement
Dr. Zarate is listed as a coinventor on a patent for the use of ketamine in major depression and suicidal ideation. Drs. Zarate and Moaddel are listed as coinventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Drs. Zarate, Moaddel, and Gould are listed as co-inventors on a patent application for the crystal forms and methods of synthesis of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine and the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. Drs. Zarate and Moaddel have assigned their patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Gould has assigned his patent rights to the University of Maryland. All other authors have no conflict of interest to disclose, financial or otherwise. Clinical Trials Registration: NCT03065335.
Figures






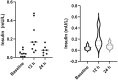
Similar articles
-
Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression.Br J Clin Pharmacol. 2012 Aug;74(2):304-14. doi: 10.1111/j.1365-2125.2012.04198.x. Br J Clin Pharmacol. 2012. PMID: 22295895 Free PMC article. Clinical Trial.
-
Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression.Biol Psychiatry. 2012 Aug 15;72(4):331-8. doi: 10.1016/j.biopsych.2012.03.004. Epub 2012 Apr 18. Biol Psychiatry. 2012. PMID: 22516044 Free PMC article.
-
Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters.J Pharmacol Exp Ther. 2016 Oct;359(1):159-70. doi: 10.1124/jpet.116.235838. Epub 2016 Jul 28. J Pharmacol Exp Ther. 2016. PMID: 27469513 Free PMC article.
-
Hydroxynorketamines: Pharmacology and Potential Therapeutic Applications.Pharmacol Rev. 2021 Apr;73(2):763-791. doi: 10.1124/pharmrev.120.000149. Pharmacol Rev. 2021. PMID: 33674359 Free PMC article. Review.
-
CYP 450 enzymes influence (R,S)-ketamine brain delivery and its antidepressant activity.Neuropharmacology. 2022 Mar 15;206:108936. doi: 10.1016/j.neuropharm.2021.108936. Epub 2021 Dec 26. Neuropharmacology. 2022. PMID: 34965407 Review.
Cited by
-
A Pilot Proteomic Analysis of Huntington's Disease by Functional Capacity.Brain Sci. 2025 Jan 16;15(1):76. doi: 10.3390/brainsci15010076. Brain Sci. 2025. PMID: 39851443 Free PMC article.
-
The Causal Association Analysis between Depression and Cerebrospinal Fluid: From the Perspective of Mendelian Randomization.Psychol Res Behav Manag. 2025 May 5;18:1085-1097. doi: 10.2147/PRBM.S508610. eCollection 2025. Psychol Res Behav Manag. 2025. PMID: 40352659 Free PMC article.
-
A Phase 1 Assessment of the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of (2R,6R)-Hydroxynorketamine in Healthy Volunteers.Clin Pharmacol Ther. 2024 Nov;116(5):1314-1324. doi: 10.1002/cpt.3391. Epub 2024 Jul 25. Clin Pharmacol Ther. 2024. PMID: 39054770 Clinical Trial.
-
Serum Proteomic and Metabolomic Signatures of High Versus Low Physical Function in Octogenarians.Aging Cell. 2025 Mar 10;24(5):e70002. doi: 10.1111/acel.70002. Online ahead of print. Aging Cell. 2025. PMID: 40059508 Free PMC article.
References
-
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. - PubMed
-
- Diazgranados N., Ibrahim L., Brutsche N.E., Newberg A., Kronstein P., Khalife S., Kammerer W.A., Quezado Z., Luckenbaugh D.A., Salvadore G., et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry. 2010;67:793–802. - PMC - PubMed
-
- Zarate C.A., Jr., Brutsche N.E., Ibrahim L., Franco-Chaves J., Diazgranados N., Cravchik A., Selter J., Marquardt C.A., Liberty V., Luckenbaugh D.A. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry. 2012;71:939–946. - PMC - PubMed
-
- Fava M., Freeman M.P., Flynn M., Judge H., Hoeppner B.B., Cusin C., Ionescu D.F., Mathew S.J., Chang L.C., Iosifescu D.V., et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD) Mol. Psychiatry. 2020;25:1592–1603. - PMC - PubMed
-
- Murrough J.W., Iosifescu D.V., Chang L.C., Al Jurdi R.K., Green C.E., Perez A.M., Iqbal S., Pillemer S., Foulkes A., Shah A., et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry. 2013;170:1134–1142. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

