Global blood miRNA profiling unravels early signatures of immunogenicity of Ebola vaccine rVSVΔG-ZEBOV-GP
- PMID: 38162033
- PMCID: PMC10755791
- DOI: 10.1016/j.isci.2023.108574
Global blood miRNA profiling unravels early signatures of immunogenicity of Ebola vaccine rVSVΔG-ZEBOV-GP
Abstract
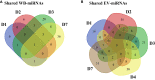
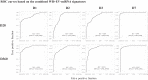
The vectored Ebola vaccine rVSVΔG-ZEBOV-GP elicits protection against Ebola Virus Disease (EVD). In a study of forty-eight healthy adult volunteers who received either the rVSVΔG-ZEBOV-GP vaccine or placebo, we profiled intracellular microRNAs (miRNAs) from whole blood cells (WB) and circulating miRNAs from serum-derived extracellular vesicles (EV) at baseline and longitudinally following vaccination. Further, we identified early miRNA signatures associated with ZEBOV-specific IgG antibody responses at baseline and up to one year post-vaccination, and pinpointed target mRNA transcripts and pathways correlated to miRNAs whose expression was altered after vaccination by using systems biology approaches. Several miRNAs were differentially expressed (DE) and miRNA signatures predicted high or low IgG ZEBOV-specific antibody levels with high classification performance. The top miRNA discriminators were WB-miR-6810, EV-miR-7151-3p, and EV-miR-4426. An eight-miRNA antibody predictive signature was associated with immune-related target mRNAs and pathways. These findings provide valuable insights into early blood biomarkers associated with rVSVΔG-ZEBOV-GP vaccine-induced IgG antibody responses.
Keywords: Health sciences; Immunology; Molecular biology; Virology.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Kanapathipillai R., Henao Restrepo A.M., Fast P., Wood D., Dye C., Kieny M.P., Moorthy V. Ebola vaccine--an urgent international priority. N. Engl. J. Med. 2014;371:2249–2251. - PubMed
-
- Huttner A., Dayer J.A., Yerly S., Combescure C., Auderset F., Desmeules J., Eickmann M., Finckh A., Goncalves A.R., Hooper J.W., et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2015;15:1156–1166. - PMC - PubMed
-
- Gsell P.S., Camacho A., Kucharski A.J., Watson C.H., Bagayoko A., Nadlaou S.D., Dean N.E., Diallo A., Diallo A., Honora D.A., et al. Ring vaccination with rVSV-ZEBOV under expanded access in response to an outbreak of Ebola virus disease in Guinea, 2016: an operational and vaccine safety report. Lancet Infect. Dis. 2017;17:1276–1284. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

