Degradation kinetics of medium chain length Polyhydroxyalkanoate degrading enzyme: a quartz crystal microbalance study
- PMID: 38162181
- PMCID: PMC10756687
- DOI: 10.3389/fbioe.2023.1303267
Degradation kinetics of medium chain length Polyhydroxyalkanoate degrading enzyme: a quartz crystal microbalance study
Abstract
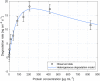
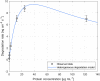
This study investigates the enzymatic degradation processes of different classes of polyhydroxyalkanoates (PHAs), a group of biopolymers naturally synthesized by various microorganisms. Medium chain length PHAs (mcl-PHAs) are distinguished biopolymers due to their biodegradability and diverse material properties. Using quartz crystal microbalance measurements as a valuable tool for accurate real-time monitoring of the enzymatic degradation process, the research provides detailed kinetic data, describing the interaction between enzymes and substrates during the enzymatic degradation process. Thin films of poly-3-hydroxybutyrate (PHB) and polyhydroxyoctanoate copolymer (PHO), containing molar fractions of about 84% 3-hydroxyoctanoate and 16% 3-hydroxyhexanoate, were exposed to scl-depolymerases from Pseudomonas lemoignei LMG 2207 and recombinant mcl-depolymerase produced in Escherichia coli DH5α harboring the plasmid pMAD8, respectively. Analyses based on a heterogeneous kinetic model for the polymer degradation indicated a six-fold stronger adsorption equilibrium constant of mcl-depolymerase to PHO. Conversely, the degradation rate constant was approximately twice as high for scl-depolymerases acting on PHB. Finally, the study highlights the differences in enzyme-substrate interactions and degradation mechanisms between the investigated scl- and mcl-PHAs.
Keywords: biodegradable polymers; degradation kinetics; depolymerase enzymes; enzymatic degradation; polyhydroxyalkanoates; quartz crystal microbalance.
Copyright © 2023 Millan and Hanik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Poly(3-hydroxyvalerate) depolymerase of Pseudomonas lemoignei.Appl Environ Microbiol. 2000 Apr;66(4):1385-92. doi: 10.1128/AEM.66.4.1385-1392.2000. Appl Environ Microbiol. 2000. PMID: 10742216 Free PMC article.
-
Colonization and degradation of polyhydroxyalkanoates by lipase-producing bacteria.Can J Microbiol. 2019 Jun;65(6):461-475. doi: 10.1139/cjm-2019-0042. Epub 2019 Mar 21. Can J Microbiol. 2019. PMID: 30897336
-
New model compounds for the efficient colorimetric screening of medium chain length polyhydroxyalkanoate (mcl-PHA) depolymerases reveal mechanism of activity.Int J Biol Macromol. 2024 Dec;283(Pt 3):137672. doi: 10.1016/j.ijbiomac.2024.137672. Epub 2024 Nov 19. Int J Biol Macromol. 2024. PMID: 39566772
-
Biosynthesis, modification, and biodegradation of bacterial medium-chain-length polyhydroxyalkanoates.J Microbiol. 2007 Apr;45(2):87-97. J Microbiol. 2007. PMID: 17483792 Review.
-
Current trends in polyhydroxyalkanoates (PHAs) biosynthesis: insights from the recombinant Escherichia coli.J Biotechnol. 2014 Jun 20;180:52-65. doi: 10.1016/j.jbiotec.2014.03.020. Epub 2014 Mar 31. J Biotechnol. 2014. PMID: 24698847 Review.
Cited by
-
Biodegradation of polyhydroxyalkanoates: current state and future prospects.Front Microbiol. 2025 Feb 24;16:1542468. doi: 10.3389/fmicb.2025.1542468. eCollection 2025. Front Microbiol. 2025. PMID: 40066265 Free PMC article. Review.
References
-
- Brigham C. J., Sinskey A. J. (2012). Applications of polyhydroxyalkanoates in the medical industry. Int. J. Biotechnol. Wellness Industries 1, 52–60. 10.6000/1927-3037.2012.01.01.03 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

