Effects of exogenous plant regulators on growth and development of "Kyoho" grape under salt alkali stress
- PMID: 38162314
- PMCID: PMC10756669
- DOI: 10.3389/fpls.2023.1274684
Effects of exogenous plant regulators on growth and development of "Kyoho" grape under salt alkali stress
Abstract
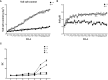
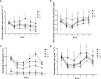
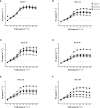
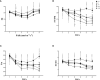
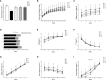
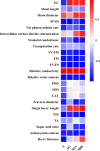
Salinity is one of the major abiotic stresses besides drought and cold stress. The application of plant growth regulators (PGRs) is an effective method to mitigate yield losses caused by salinity. However, we investigated the effects of exogenous regulatory substances (γ-aminobutyric acid (GABA), salicylic acid (SA), and brassinolide (BR) on the growth and development of "Kyoho" grapevine under salt stress. The results showed that exogenous regulators GABA, SA, and BR alleviated the inhibition of grape growth by saline stress and regulated the effects of salinity stress on grape fruit development and quality. All three regulators significantly increased fruit set, cross-sectional diameter, weight per unit, and anthocyanin content. In conclusion, this study provides a theoretical basis for grape production practices by using exogenous aminobutyric acid (GABA), salicylic acid (SA), and brassinolide (BR) to mitigate the hazards of salinity stress.
Keywords: brassinolide; fruit quality; grapevine growth; salicylic acid; salt alkali stress; γ-aminobutyric acid.
Copyright © 2023 Zhao, Li, Shi, Sanaullah Malik, Quan, Guo, Wang and Wang.
Conflict of interest statement
Author XS was employed by company Sinochem Agriculture Holdings. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Root-applied brassinosteroid and salicylic acid enhance thermotolerance and fruit quality in heat-stressed 'Kyoho' grapevines.Front Plant Sci. 2025 Apr 3;16:1563270. doi: 10.3389/fpls.2025.1563270. eCollection 2025. Front Plant Sci. 2025. PMID: 40247944 Free PMC article.
-
Improving Performance of Salt-Grown Crops by Exogenous Application of Plant Growth Regulators.Biomolecules. 2021 May 24;11(6):788. doi: 10.3390/biom11060788. Biomolecules. 2021. PMID: 34073871 Free PMC article. Review.
-
Exogenous γ-aminobutyric acid improves the photosynthesis efficiency, soluble sugar contents, and mineral nutrients in pomegranate plants exposed to drought, salinity, and drought-salinity stresses.BMC Plant Biol. 2023 Nov 6;23(1):543. doi: 10.1186/s12870-023-04568-2. BMC Plant Biol. 2023. PMID: 37926819 Free PMC article.
-
Effect of plant growth regulators and deficit irrigation on canopy traits, yield, water productivity and fruit quality of eggplant (Solanum melongena L.) grown in the water scarce environment.J Environ Manage. 2020 May 15;262:110320. doi: 10.1016/j.jenvman.2020.110320. Epub 2020 Feb 29. J Environ Manage. 2020. PMID: 32250803
-
Emerging Roles of Salicylic Acid in Plant Saline Stress Tolerance.Int J Mol Sci. 2023 Feb 8;24(4):3388. doi: 10.3390/ijms24043388. Int J Mol Sci. 2023. PMID: 36834798 Free PMC article. Review.
Cited by
-
Metabolome and Transcriptome Analysis Revealed the Pivotal Role of Exogenous Melatonin in Enhancing Salt Tolerance in Vitis vinifera L.Int J Mol Sci. 2024 Mar 25;25(7):3651. doi: 10.3390/ijms25073651. Int J Mol Sci. 2024. PMID: 38612463 Free PMC article.
-
Taurine priming improves redox balance, osmotic adjustment, and nutrient acquisition to lessen phytotoxic effects of neutral and alkaline salts on pea (Pisum sativum L.).Plant Signal Behav. 2025 Dec;20(1):2480224. doi: 10.1080/15592324.2025.2480224. Epub 2025 Mar 25. Plant Signal Behav. 2025. PMID: 40133225 Free PMC article.
-
Relationship between the GABA Pathway and Signaling of Other Regulatory Molecules.Int J Mol Sci. 2024 Oct 6;25(19):10749. doi: 10.3390/ijms251910749. Int J Mol Sci. 2024. PMID: 39409078 Free PMC article. Review.
-
Exogenous melatonin delays leaves senescence and enhances saline and alkaline stress tolerance in grape seedlings.Plant Signal Behav. 2024 Dec 31;19(1):2334511. doi: 10.1080/15592324.2024.2334511. Epub 2024 Apr 22. Plant Signal Behav. 2024. PMID: 38650457 Free PMC article.
References
LinkOut - more resources
Full Text Sources

